Voxzogo
Generic name: vosoritide
Dosage form: injection, for subcutaneous use
Medically reviewed by A Ras MD. Last updated April 11, 2022
What is Voxzogo?
Voxzogo is a prescription drug used to help children with achondroplasia who are 5 years old or older and have open bone growth plates grow more linearly (epiphyses).
It is unknown whether VOXZOGO is safe and effective in children under the age of five who have achondroplasia.
DESCRIPTION
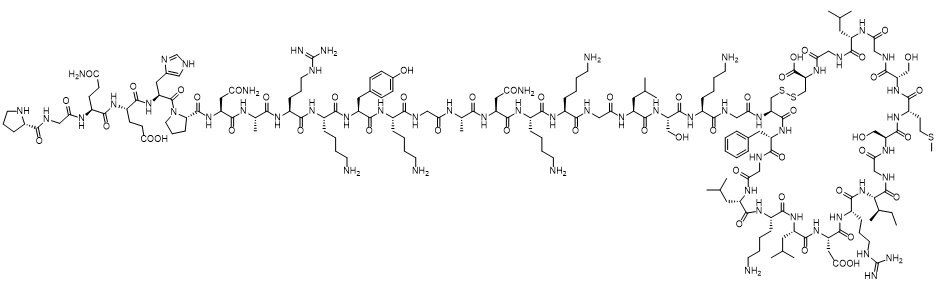
VOXZOGO contains vosoritide, a human C type natriuretic peptide (CNP) analog. Vosoritide is a 39 amino acid peptide. Its amino acid sequence includes the 37 C terminal amino acids of the human CNP53 sequence plus Pro Gly on the N terminus to convey resistance to neutral endopeptidase (NEP) degradation. Vosoritide is manufactured from Escherichia coli using recombinant DNA technology. Vosoritide has a chemical formula of C176H290N56O51S3 with a molecular weight of 4.1 kDa.
Vosoritide has the structural formula shown in Figure 1.
Figure 1
VOXZOGO (vosoritide) for injection, is a sterile, preservative-free white-to-yellow lyophilized powder, for subcutaneous administration after reconstitution with Sterile Water for Injection, USP.
VOXZOGO is provided as a single-dose vial containing 0.4 mg, 0.56 mg, or 1.2 mg of vosoritide per vial. A pre-filled syringe containing Sterile Water for Injection, USP for use as a diluent is also provided. The contents of each single dose vial are summarized by strength in Table 3. The product contains no preservative.
| Strength | Inactive Ingredients per Vial |
|---|---|
| Trehalose dihydrate and D-Mannitol are used as isotonic agent. Citric acid monohydrate and sodium citrate dihydrate are used as buffering agent. | |
| VOXZOGO 0.4 mg | Trehalose dihydrate (29.01 mg), mannitol (7.5 mg), sodium citrate dihydrate (0.54 mg), methionine (0.36 mg), citric acid monohydrate (0.14 mg), and polysorbate 80 (0.025 mg). After reconstitution with 0.5 mL Sterile Water for Injection USP, the resulting concentration is 0.4 mg/0.5 mL of vosoritide and the nominal deliverable volume is 0.4 mL. |
| VOXZOGO 0.56 mg | Trehalose dihydrate (40.61 mg), mannitol (10.50 mg), sodium citrate dihydrate (0.76 mg), methionine (0.51 mg), citric acid monohydrate (0.20 mg), and polysorbate 80 (0.035 mg). After reconstitution with 0.7 mL Sterile Water for Injection USP, the resulting concentration is 0.56 mg/0.7 mL of vosoritide and the nominal deliverable volume is 0.6 mL. |
| VOXZOGO 1.2 mg | Trehalose dihydrate (34.81 mg), mannitol (9 mg), sodium citrate dihydrate (0.65 mg), methionine (0.44 mg), citric acid monohydrate (0.17 mg), and polysorbate 80 (0.030 mg). After reconstitution with 0.6 mL Sterile Water for Injection USP, the resulting concentration is 1.2 mg/0.6 mL of vosoritide and the nominal deliverable volume is 0.5 mL. |
1 INDICATIONS AND USAGE
VOXZOGO is indicated to increase linear growth in pediatric patients with achondroplasia who are 5 years of age and older with open epiphyses. This indication is approved under accelerated approval based on an improvement in annualized growth velocity [see CLINICAL STUDIES (14)]. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trial(s).¶
2 DOSAGE AND ADMINISTRATION
2.1 Important Instructions Prior to Administration of VOXZOGO
To reduce the risk of low blood pressure and its associated signs and symptoms, instruct the caregiver and patient that the patient should [see WARNINGS AND PRECAUTIONS (5.1)]:
- Have adequate food intake prior to VOXZOGO administration.
- Drink approximately 240-300 mL of fluid in the hour prior to VOXZOGO administration.
2.2 Recommended Dosage and Administration
The recommended dosage of VOXZOGO is based on the patient’s actual body weight (see TABLE 1). VOXZOGO is administered by subcutaneous injection once daily [see DOSAGE AND ADMINISTRATION (2.4)].
Inject VOXZOGO at approximately the same time each day, if possible. The volume of VOXZOGO to be administered (injection volume) is based on the patient’s actual body weight and the concentration of reconstituted VOXZOGO (0.8 mg/mL or 2 mg/mL) (Table 1). VOXZOGO must be reconstituted prior to use [see DOSAGE AND ADMINISTRATION (2.4)].
| Actual Body Weight | Vial Strength for Reconstitution* | Dose | Injection Volume |
|---|---|---|---|
|
|||
| 10-11 kg | 0.4 mg | 0.24 mg | 0.3 mL |
| 12-16 kg | 0.56 mg | 0.28 mg | 0.35 mL |
| 17-21 kg | 0.56 mg | 0.32 mg | 0.4 mL |
| 22-32 kg | 0.56 mg | 0.4 mg | 0.5 mL |
| 33-43 kg | 1.2 mg | 0.5 mg | 0.25 mL |
| 44-59 kg | 1.2 mg | 0.6 mg | 0.3 mL |
| 60-89 kg | 1.2 mg | 0.7 mg | 0.35 mL |
| ≥90 kg | 1.2 mg | 0.8 mg | 0.4 mL |
Missed dose
If a dose of VOXZOGO is missed, it can be administered within 12 hours of the scheduled time of administration. Beyond 12 hours, skip the missed dose and administer the next daily dose according to the usual dosing schedule.
2.3 Growth Monitoring
Monitor and assess patient body weight, growth, and physical development regularly every 3-6 months. Adjust the dosage according to the patient’s actual body weight [see DOSAGE AND ADMINISTRATION (2.2)].
Permanently discontinue VOXZOGO upon confirmation of no further growth potential, indicated by closure of epiphyses.
2.4 Preparation and Administration
Reconstitute VOXZOGO before administration using the provided diluent syringe containing Sterile Water for Injection, USP (see RECONSTITUTION INSTRUCTIONS below).
Caregivers may inject VOXZOGO subcutaneously after proper training by a healthcare professional on the preparation and administration of VOXZOGO [see INSTRUCTIONS FOR USE].
Reconstitution Instructions
- Select the correct VOXZOGO strength and prefilled diluent syringe co-pack based on the patient’s actual body weight [see DOSAGE AND ADMINISTRATION (2.2)].
- Remove VOXZOGO vial and prefilled diluent syringe (Sterile Water for Injection, USP) from the refrigerator and allow the vial and prefilled diluent syringe to reach room temperature before reconstituting VOXZOGO.
- Attach the diluent needle provided with ancillary supplies to the diluent prefilled syringe.
- Inject the entire diluent prefilled syringe volume into the vial.
- Gently swirl the diluent in the vial until the white powder is completely dissolved. Do not shake.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit. Once reconstituted VOXZOGO is a clear, colorless to yellow liquid. The solution should not be used if discolored or cloudy, or if particles are present. The concentration of reconstituted solution is 0.8 mg/mL or 2.0 mg/mL.
- After reconstitution, VOXZOGO can be held in the vial at a room temperature 20°C to 25°C (68°F to 77°F) for a maximum of 3 hours.
- For administration, extract the required dose volume from the vial using the supplied administration syringe [see DOSAGE AND ADMINISTRATION (2.2)].
Discard any unused portion. Do not pool unused portions from the vials. Do not administer more than 1 dose from a vial. Do not mix with other medications.
Instructions for Subcutaneous Administration
See the INSTRUCTIONS FOR USE document for detailed, illustrated instructions.
- Ensure patients have had adequate food and fluid intake prior to VOXZOGO administration [see DOSAGE AND ADMINISTRATION (2.1)]. Slowly withdraw the dosing volume of the reconstituted VOXZOGO solution from the single-dose vial into a syringe.
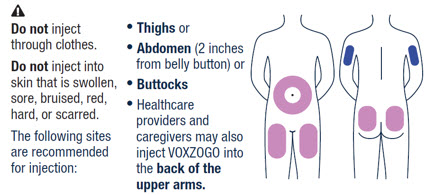
- Rotate sites for subcutaneous injections.
- The recommended injection sites for VOXZOGO are: the front middle of the thighs, the lower part of the abdomen at least 2 inches (5 centimeters) away from the navel, top of the buttocks or the back of the upper arms. The same injection area should not be used on two consecutive days. Do not inject VOXZOGO into sites that are red, swollen, or tender.
3 DOSAGE FORMS AND STRENGTHS
For Injection: 0.4 mg, 0.56 mg, or 1.2 mg as a white to yellow lyophilized powder for reconstitution in a single-dose vial.
4 CONTRAINDICATIONS(WHAT IS THIS?)
None.
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Low Blood Pressure
Transient decreases in blood pressure were observed in clinical studies of VOXZOGO. Subjects with significant cardiac or vascular disease and patients on anti-hypertensive medicinal products were excluded from participation in VOXZOGO clinical trials. To reduce the risk of a decrease in blood pressure and associated symptoms (dizziness, fatigue and/or nausea), instruct patients to be well hydrated and have adequate food intake prior to administration of VOXZOGO [see DOSAGE AND ADMINISTRATION (2.1) and ADVERSE REACTIONS (6.1)].
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Risk of Low Blood Pressure [see WARNINGS AND PRECAUTIONS (5.1)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
VOXZOGO was studied in a 52-week, randomized, double-blind, placebo-controlled trial in 121 subjects with achondroplasia (Study 1) [see CLINICAL STUDIES (14)].
The subjects’ ages ranged from 5.1 to 14.9 years with a mean of 8.7 years. Sixty four (53%) subjects were male and 57 (47%) were female. Overall, 86 (71%) subjects were White, 23 (19%) were Asian, 5 (4%) were Black or African American, and 7 (6%) were classified as “multiple” race. The demographic and baseline characteristics were balanced between treatment groups. The subjects received either VOXZOGO 15 mcg/kg, or placebo administered subcutaneously once daily.
Table 2 shows adverse reactions that occurred in ≥5% of patients treated with VOXZOGO and at a percentage greater than placebo.
| Adverse Reaction | Placebo (N=61) n (%) |
VOXZOGO (N=60) n (%) |
|---|---|---|
| Abreviations: N, total number of subjects in the treatment arm; n, number of subjects with the adverse reaction; %, percent of subjects with the adverse reaction. | ||
|
||
| Injection site erythema | 42 (69%) | 45 (75%) |
| Injection site swelling | 22 (36%) | 37 (62%) |
| Vomiting | 12 (20%) | 16 (27%) |
| Injection site urticaria | 6 (10%) | 15 (25%) |
| Arthralgia | 4 (7%) | 9 (15%) |
| Decreased blood pressure | 3 (5%) | 8 (13%) |
| Gastroenteritis† | 5 (8%) | 8 (13%) |
| Diarrhea | 2 (3%) | 6 (10%) |
| Dizziness‡ | 2 (3%) | 6 (10%) |
| Ear pain | 3 (5%) | 6 (10%) |
| Influenza | 3 (5%) | 6 (10%) |
| Fatigue§ | 2 (3%) | 5 (8%) |
| Seasonal allergy | 1 (2%) | 4 (7%) |
| Dry skin | 0 | 3 (5%) |
Discussion of Selected Adverse Reactions
Decreased blood pressure
Eight (13%) of 60 subjects treated with VOXZOGO had a total of 11 events of transient decrease in blood pressure compared to 3 (5%) of 61 subjects on placebo, identified predominantly during periods of frequent monitoring at clinical visits after dosing over a 52-week treatment period. The median time to onset from injection was 31 (18 to 120) minutes with resolution within 31 (5 to 90) minutes in VOXZOGO-treated subjects. Two out of 60 (3%) VOXZOGO-treated subjects each had one symptomatic episode of decreased blood pressure with vomiting and/or dizziness compared to 0 of 61 (0%) subjects on placebo.
Injection site reactions
Injection site reactions occurred in 51 (85%) subjects receiving VOXZOGO and 50 (82%) subjects receiving placebo over a 52 week period of treatment. Injection site reactions included the preferred terms injection site erythema, injection site reaction, injection site swelling, injection site urticaria, injection site pain, injection site bruising, injection site pruritus, injection site hemorrhage, injection site discoloration, and injection site induration. Over a 52 week period, 51 (85%) of 60 subjects receiving VOXZOGO experienced a total of 6983 events of injection site reactions, while 50 (82%) of 61 subjects receiving placebo experienced a total of 1776 events of injections site reactions, representing 120.4 events per person/year exposure and 29.2 per person/year exposure, respectively. One injection site reaction event could have been associated with one or more injection site reaction symptoms (e.g., injection site swelling, injection site erythema, injection site urticaria, etc.). Two subjects in the VOXZOGO arm discontinued treatment due to adverse reactions of pain and anxiety with injections.
6.2 Immunogenicity
As with all peptides, there is potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies in the studies described below with the incidence of antibodies in other studies or to other products may be misleading.
Of 131 subjects who were treated with VOXZOGO 15 mcg/kg/day and evaluable for the presence of anti-drug antibodies (ADA) for up to 240 weeks, ADA were detected in 35% (46/131). The earliest time to ADA development was day 85. All ADA-positive subjects tested negative for anti-vosoritide neutralizing antibodies. There was no correlation between the number, duration, or severity of hypersensitivity adverse reactions or injection site reactions and ADA positivity or mean ADA titer. There was no association between ADA positivity or mean ADA titer and change from baseline in annual growth velocity (AGV) or height Z-score at month 12. There was no impact of serum ADA detected on the plasma PK measurements of vosoritide.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data on vosoritide use in pregnant women to evaluate for a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. In animal reproduction studies, there was no evidence of embryo-fetal toxicity or congenital malformations when pregnant rats and rabbits were administered vosoritide subcutaneously at doses equivalent to 14-times and 200-times, respectively, the exposure at the maximum recommended human dose (MRHD) (see DATA).
The estimated background risk of major birth defects for the indicated population is higher than the general population. The estimated background risk of miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Animal Data
In an embryofetal developmental toxicity study in rats, vosoritide was administered at 90, 270, 540 mcg/kg once daily by subcutaneous injection during the period of major organogenesis from gestation day (GD) 6 – 17. There were no effects on maternal animals or on embryofetal development at the highest dose administered (14-times the exposure at the MRHD).
In an embryofetal developmental toxicity study in rabbits, vosoritide was administered at 45, 135, 240 mcg/kg once daily by subcutaneous injection during the period of major organogenesis (GD 7 – 19). No effects were observed in maternal animals or on embryofetal development at the highest dose administered (200-times the exposure at the MRHD).
In a pre- and postnatal toxicity study in rats, vosoritide was administered at 90, 270, and 540 mcg/kg once daily by subcutaneous injection during the period of major organogenesis and continuing to weaning (GD 6 through postpartum day 20). There were no effects on maternal animals, including maintenance of pregnancy, parturition, or care of offspring, and no effects were noted on offspring growth and development or ability to reproduce at the highest dose (14-times the exposure at the MRHD).
8.2 Lactation
Risk Summary
There is no information regarding the presence of vosoritide in human milk, the effects on the breastfed infant, or the effects on milk production. Vosoritide is present in rat milk. When a drug is present in animal milk, it is likely that the drug will be present in human milk. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for VOXZOGO and any potential adverse effects on the breastfed child from VOXZOGO or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of VOXZOGO have been established in pediatric patients aged 5 years and older for the improvement in linear growth in patients with achondroplasia. Use of VOXZOGO for this indication is supported by evidence from adequate and well-controlled studies in pediatric patients aged 5 years and older [see ADVERSE REACTIONS (6.1), CLINICAL PHARMACOLOGY (12.3), and CLINICAL STUDIES (14)].
Safety and effectiveness of VOXZOGO in pediatric patients with achondroplasia below the age of 5 years have not been established.
8.6 Renal Impairment
The influence of renal impairment on the pharmacokinetics of VOXZOGO has not been evaluated. No dosage adjustment is needed for patients with eGFR ≥ 60 mL/min/1.73 m2. VOXZOGO is not recommended for patients with eGFR < 60 mL/min/1.73 m2.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
In patients with achondroplasia, endochondral bone growth is negatively regulated due to a gain of function mutation in fibroblast growth factor receptor 3 (FGFR3). Binding of vosoritide to natriuretic peptide receptor-B (NPR-B) antagonizes FGFR3 downstream signaling by inhibiting the extracellular signal-regulated kinases 1 and 2 (ERK1/2) in the mitogen-activated protein kinase (MAPK) pathway at the level of rapidly accelerating fibrosarcoma serine/threonine protein kinase (RAF-1). As a result, vosoritide, like CNP, acts as a positive regulator of endochondral bone growth as it promotes chondrocyte proliferation and differentiation.
In animal models with open growth plates, vosoritide administration resulted in the promotion of chondrocyte proliferation and differentiation that led to a widening of the growth plate and subsequent increase in skeletal growth. In the mouse models of FGFR3-related chondrodysplasia, a partial or complete normalization of the dwarfism phenotype was observed.
12.2 Pharmacodynamics
NPR-B Binding Activity Biomarker and Bone Metabolism Biomarker
An increase in urinary cyclic guanosine monophosphate (cGMP) concentrations from pre-dose baseline were observed within the first four hours post-dose, with a maximum level at 2 hours post-dose, after VOXZOGO administration to pediatric patients with achondroplasia.
Daily administration of VOXZOGO also led to the increase from baseline in serum collagen type X marker (CXM), an endochondral ossification biomarker and remains elevated beyond 24 months. In subjects aged 5 – 14 years old at screening, exposure-response analyses showed that vosoritide activity measured by urinary cGMP was near saturation at the dose of 15 mcg/kg once daily, while maximal increase in growth plate activity indicated by CXM was achieved at this dose.
Cardiac Electrophysiology
At the maximum approved recommended dose, Voxzogo does not prolong the QT interval to any clinically relevant extent.
12.3 Pharmacokinetics
The area under the concentration-time curve (AUC) and peak concentration (Cmax) of vosoritide increased greater than proportionally following subcutaneous administration to pediatric subjects with achondroplasia in the dose range of 7.5 to 30.0 mcg/kg. The pharmacokinetics of vosoritide were evaluated in 58 subjects aged 5 to 13 years with achondroplasia who received subcutaneous injections of vosoritide 15 mcg/kg once daily for 52 weeks. The mean (± SD) Cmax and area under the concentration-time curve from time zero to the last measurable concentration (AUC0-t) observed across 52 weeks of treatment ranged from 4.71 (± 2.32) to 7.18 (± 9.65) ng/mL, and 161 (± 98.1) to 290 (± 235) ng-min/mL, respectively. No drug accumulation was observed following 15 mcg/kg once daily dosing. The exposure of vosoritide increased with the duration of treatment. The mean AUC0-t at week 52 increased approximately 20% compared to that at day 1.
Absorption
Absolute bioavailability for vosoritide following subcutaneous injection was not determined. Vosoritide was absorbed with a median Tmax of 15 minutes after dosing.
Distribution
The mean (± SD) apparent volume of distribution of vosoritide across 52 weeks of subcutaneous administration of VOXZOGO 15 mcg/kg once daily ranged from 2880 (± 2450) to 3020 (± 1980) mL/kg.
Elimination
The mean (± SD) apparent clearance of vosoritide across 52 weeks of subcutaneous administration of VOXZOGO 15 mcg/kg once daily ranged from 79.4 (± 53.0) to 104 (± 98.8) mL/min/kg. The mean (± SD) half-life ranged from 21.0 (± 4.7) to 27.9 (± 9.9) minutes.
Metabolism
The metabolism of vosoritide is expected to occur via catabolic pathways with degradation into small peptide fragments and amino acids.
Special Populations
No clinically significant differences in the vosoritide pharmacokinetics were observed based on age (0.9 to 16 years), sex or race. The effect of hepatic or renal impairment on the pharmacokinetics of vosoritide is unknown.
Body weight
Population pharmacokinetic analyses indicated that body weight is a significant covariate for vosoritide clearance and volume of distribution. The apparent clearance and volume of distribution of vosoritide increased with increasing body weight in patients with achondroplasia (9 to 74.5 kg).
Drug Interaction Studies
In vitro assessment of drug-drug interactions
In vitro studies showed that vosoritide, at therapeutic concentrations, does not inhibit or induce Cytochrome P450 enzymes.
In vivo assessment of drug-drug interactions
No clinical studies evaluating the drug-drug interaction potential of vosoritide have been conducted.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long term carcinogenicity studies and genotoxicity studies with vosoritide have not been performed.
In a fertility and reproductive study in male and female rats at doses up to 540 mcg/kg/day (15-times the exposure at the MRHD), vosoritide had no effect on mating performance, fertility, or litter characteristics.
14 CLINICAL STUDIES
The safety and effectiveness of VOXZOGO in patients with achondroplasia were assessed in one 52-week, multi-center, randomized, double-blind, placebo-controlled, phase 3 study – Study 1 (NCT03197766).
Study 1 was conducted in 121 subjects with genetically-confirmed achondroplasia, who were randomized to either VOXZOGO (N=60) or placebo (N=61). The dosage of VOXZOGO was 15 mcg/kg administered subcutaneously once daily. Baseline standing height, weight Z-score, body mass index (BMI) Z-score, and upper to lower body ratio were collected for at least 6 months prior to randomization. Subjects with limb-lengthening surgery in the prior 18 months or who planned to have limb-lengthening surgery during the study period were excluded. The study included a 52-week placebo-controlled treatment phase followed by an open-label treatment extension study period in which all subjects received VOXZOGO. The primary efficacy endpoint was the change from baseline in annualized growth velocity (AGV) at Week 52 compared with placebo.
The subjects’ ages ranged from 5.1 to 14.9 years with a mean of 8.7 years. Sixty four (53%) subjects were male and 57 (47%) were female. Overall, 86 (71%) subjects were White, 23 (19%) were Asian, 5 (4%) were Black or African American, and 7 (6%) were classified as “multiple” race. The subjects had a mean baseline height standard deviation score (SDS) of -5.13.
Treatment with VOXZOGO for 52 weeks resulted in a treatment difference in the change from baseline in AGV of 1.57 cm/year after 52 weeks of treatment (Table 4).
| Placebo (N=61*) |
VOXZOGO 15 mcg/kg Daily (N=60*) |
|
|---|---|---|
| Abbreviations: AGV, annualized growth velocity; 95% CI, 95% confidence interval; LS, least-square; SD, standard deviation | ||
|
||
| Baseline mean (SD)† | 4.06 (1.20) | 4.26 (1.53) |
| Change from baseline‡ | -0.17 | 1.40 |
| Difference in change of VOXZOGO – Placebo‡ (95% CI) |
1.57 (1.22, 1.93)§ |
|
The improvement in AGV in favor of VOXZOGO was consistent across all predefined subgroups analyzed including sex, age group, Tanner stage, baseline height Z-score, and baseline AGV.
Height Standard Deviation Score (SDS)
The LS mean change from baseline to Week 52 in height SDS was -0.02 in the placebo group and 0.26 in the VOXZOGO group. The difference in LS mean change from baseline was 0.28 (95% CI 0.17, 0.39; p<0.0001) in favor of VOXZOGO. The LS mean change from baseline to Week 52 in upper to lower body segment ratio was -0.02 in the placebo group and -0.03 in the VOXZOGO group. The difference in LS mean change from baseline was -0.01 (95% CI -0.05, 0.02; p=0.5).
Open-label extension
After the 52 week double blind, placebo-controlled, phase 3 study, Study 1, 58 subjects initially randomized to VOXZOGO enrolled into an open-label extension. Among the subjects who had 2 years of follow-up since randomization, the improvement in AGV was maintained.
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
VOXZOGO for injection is a white to yellow lyophilized powder for reconstitution and is provided as a co-pack which includes ten:
- Sterile, single-dose 2 mL glass vials containing VOXZOGO
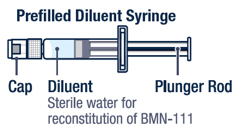
- Diluent (Sterile Water for Injection, USP) in a single-dose prefilled syringe
- Diluent transfer needles (23 gauge)
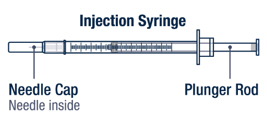
- Single-dose administration syringes (30 gauge) both with needle retraction safety devices
| Strength (mg) | Diluent (mL) | Co-pack NDC Number | Flip Cap Color |
|---|---|---|---|
| 0.4 | 0.5 | NDC 68135-082-36 | White |
| 0.56 | 0.7 | NDC 68135-119-66 | Magenta |
| 1.2 | 0.6 | NDC 68135-181-93 | Grey |
The following items to be obtained separately; alcohol aseptic wipes, gauze, bandages and sharps container.
Storage
Refrigerate VOXZOGO vials and prefilled diluent syringes at 2°C to 8°C (36°F to 46°F). Do not freeze.
VOXZOGO can be stored at room temperature 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) for 90 days. Do not return VOXZOGO to the refrigerator once stored at room temperature.
After reconstitution, VOXZOGO can be held in the vial at room temperature 20°C to 25°C (68°F to 77°F) for a maximum of 3 hours [see DOSAGE AND ADMINISTRATION (2.4)].
Record the starting date of room-temperature storage clearly on the unopened product carton.
Do not use beyond expiration date on the label.
Store in the original package to protect from light.
Handling
Reconstituted VOXZOGO must be administered within 3 hours of reconstitution [see DOSAGE AND ADMINISTRATION (2.4)].
17 PATIENT COUNSELING INFORMATION
Advise the patient and caregiver to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Preparation and Administration
Instruct caregivers on proper preparation and administration of VOXZOGO. Ensure caregivers have demonstrated the ability to perform a subcutaneous injection [see DOSAGE AND ADMINISTRATION (2.4)].
Instruct caregivers in the technique of proper syringe and needle disposal, and advise them not to reuse these items. Instruct caregivers to dispose needles and syringes in a puncture-resistant container.
Risk of Low Blood Pressure
Inform caregivers and patients that VOXZOGO may lower blood pressure after administration. Instruct caregivers and patients that prior to VOXZOGO administration, the patient should have adequate food intake and within the hour prior to administration, the patient should drink approximately 8-10 ounces (240-300 mL) of fluid [see DOSAGE AND ADMINISTRATION (2.1) and WARNINGS AND PRECAUTIONS (5.1)].
INSTRUCTIONS FOR USE VOXZOGO
This Instructions for Use contains information for caregivers on how to inject VOXZOGO.
Read these Instructions for Use before you start using VOXZOGO and each time you get a refill. There may be new information. This information does not take the place of talking to your child’s healthcare provider about your child’s medical condition and their treatment. Before you use VOXZOGO for the first time, make sure your child’s healthcare provider shows you the right way to use it. Contact your child’s healthcare provider if you or your child have any questions.
Important Information You Need to Know Before Injecting VOXZOGO
- Wash your hands with soap and water.
- Do not drop VOXZOGO or put opened items down on surfaces that are not clean.
- VOXZOGO is available in more than 1 strength. Make sure the strength matches your prescription strength. Do not open packaging until ready to use.
- Take the VOXZOGO vial and prefilled diluent syringe out of the refrigerator and allow them to reach room temperature before mixing.
- Inspect the vial and supplies for any signs of damage or contamination. Do not use if damaged or contaminated.
- Check the expiration date. The expiration date can be found on the carton, vial and prefilled diluent syringe. Do not use if expired.
- Your child should eat a meal and drink a glass (about 8 to 10 ounces) of fluid (such as water, milk, or juice) within 1 hour before injection.
- VOXZOGO should be given at about the same time every day.
- Do not mix VOXZOGO with other medicines.
- After mixing the VOXZOGO, use it right away. Do not use the mixed VOXZOGO if it has been sitting at room temperature for more than 3 hours. Throw it away (dispose of) in a sharps container. See STEP 18 and “HOW TO THROW AWAY (DISPOSE OF) VOXZOGO” for more information.
- Do not reuse any of the supplies. After the injection, throw away (dispose of) the used vial even if there is VOXZOGO remaining. See STEP 18 and “HOW TO THROW AWAY (DISPOSE OF) VOXZOGO” for more information.
How to Store VOXZOGO
- Store the VOXZOGO vial and prefilled diluent syringe in the refrigerator between 36°F to 46°F (2°C to 8°C).
- You may store VOXZOGO (before mixing) at room temperature between 68°F to 77°F (20°C to 25°C) for 90 days. Record the date you started storing VOXZOGO at room temperature on the carton to keep track of the 90 days. Do not return VOXZOGO to the refrigerator after it has been stored at room temperature. Throw VOXZOGO away if unused within 90 days of storing at room temperature.
- Do not freeze VOXZOGO.
- Store VOXZOGO out of direct sunlight.
Keep VOXZOGO and all other medicines out of the reach of children.
Supplies Needed to Inject VOXZOGO
Gather all of these supplies on a clean, flat surface before injecting.
| Items supplied | Items not supplied If you do not have these items, ask your pharmacist. |
|
VOXZOGO |
 |
|
 |
 |
|
 |
 |
|
 |
Preparing VOXZOGO for Injection
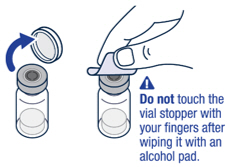
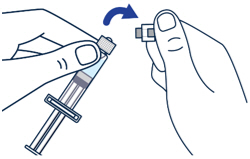
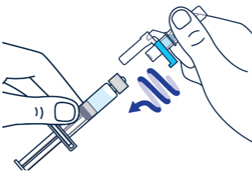
| ▶ Step 1 On a clean flat surface, flip off the vial cap and wipe the top with an alcohol pad. |
▶ Step 2 Gently bend to snap off the cap from the prefilled diluent syringe. |
▶ Step 3 Twist the diluent needle onto the prefilled diluent syringe until you can no longer twist it. Do not use the prefilled diluent syringe to give the injection. |
||
 |
 |
 |
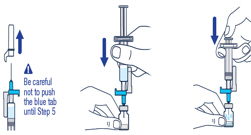
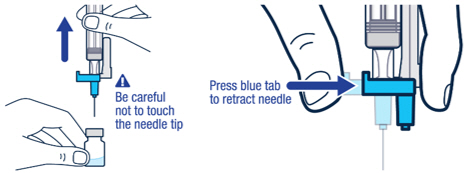
| ▶ Step 4 Pull off the needle cap and insert the needle through the middle of the vial stopper. Slowly push the plunger rod down to inject all of the liquid. |
▶ Step 5 Remove the needle from the vial, then press the blue tab for the needle to pull back (retract). Throw away the needle and syringe in a sharps container. See STEP 18 and “HOW TO THROW AWAY (DISPOSE OF) VOXZOGO.” Do not use the prefilled diluent syringe to give the injection. |
|||
 |
 |
|||
| ▶ Step 6 Gently swirl the vial until the powder has completely dissolved and the solution is clear. Do not shake. |
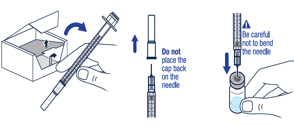
▶ Step 7 Take the injection syringe out of the carton. Pull off the needle cap from the injection syringe and insert the needle straight through the middle of the vial stopper. Be careful not to bend the needle. |
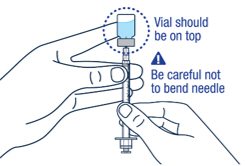
▶ Step 8 Carefully hold the vial and syringe and turn the vial upside down with the needle still inserted. The vial should be on top. Be careful not to bend the needle. |
||
 |
 |
 |
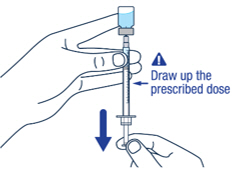
| ▶ Step 9 Keep the needle tip in the medicine and slowly pull the plunger rod back to draw up the prescribed dose in the syringe. Check the prescription label for how much to draw up. |
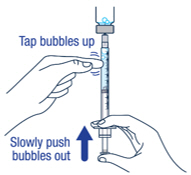
▶ Step 10 Remove large air bubbles in the syringe by gently tapping the syringe. Then push the bubbles back into the vial. |
|||
 |
 |
| ▶ Step 11 Repeat steps 9 and 10 until you have the correct prescribed dose in the syringe and no large bubbles. |
▶ Step 12 Make sure you have the prescribed dose in the syringe, then remove the vial and prepare to give the dose. |
|||
 |
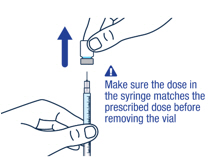 |
|||
Select and Prepare Injection Site
| ▶ Step 13 | ▶ Step 14 | |||
| VOXZOGO should be injected into the fatty layer under the skin (subcutaneous) only. Do not inject into the same site 2 times in a row. |
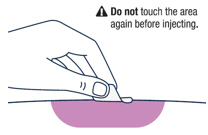
Wipe the injection site with an alcohol pad and let the skin air dry. | |||
 |
 |
|||
Giving VOXZOGO Injection
| ▶ Step 15 | ▶ Step 16 | |||
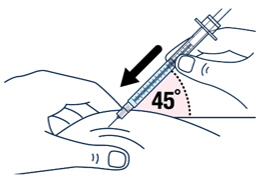
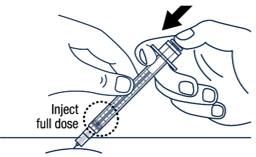
| After wiping the site with an alcohol pad, pinch the skin up around the selected injection site. | Quickly insert the needle all the way into the skin at a 45-degree angle. | |||
 |
 |
| ▶ Step 17 Release the pinch and slowly push the plunger rod all the way down. |
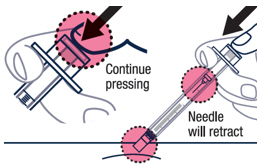
 |
Continue pressing the plunger rod until the needle retracts into the syringe. | ▶ Step 18 Throw away the used vial, syringes and needles in a sharps container. See “HOW TO THROW AWAY (DISPOSE OF) VOXZOGO” for more information. |
|
 |
 |
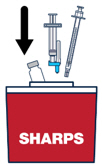 |
How to Throw Away (Dispose of) VOXZOGO
Put your used or expired vials, needles and syringes in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) the vials, loose needles and syringes in your household trash.
If you do not have a FDA-cleared sharps disposal container, you may use a household container that:
- is made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid without sharps being able to come out,
- is upright and stable during use,
- is leak-resistant, and
- is properly labeled to warn of hazardous waste inside the container.
When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be local or state laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at: http://www.fda.gov/safesharpsdisposal
Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
After the Injection
- Inspect the injection site. If a small amount of bleeding occurs from the injection site, gently press a gauze pad on it for a few seconds or apply a bandage. Do not rub the injection site.
- Monitor for signs of low blood pressure, such as dizziness, tiredness, and nausea. If your child experiences these symptoms you should call your child’s healthcare provider, then get your child to lay back with legs raised.
Label
PRINCIPAL DISPLAY PANEL
NDC 68135-082-36
Rx Only
VOXZOGO™
(vosoritide) for injection
0.4 mg per vial
For Subcutaneous Use Only
This carton contains:
– Ten single-dose vials of Voxzogo
– Ten disposable prefilled syringes
containing 0.5 mL diluent (Sterile
Water for Injection, USP) for Voxzogo
– Ten sterile, disposable needles for
prefilled diluent syringes
– Ten sterile disposable 1 mL syringes
with needles for dosing
– One Prescribing Information
– One Instructions for Use
– One Patient Information
Must be reconstituted
with diluent provided
BiOMARIN®
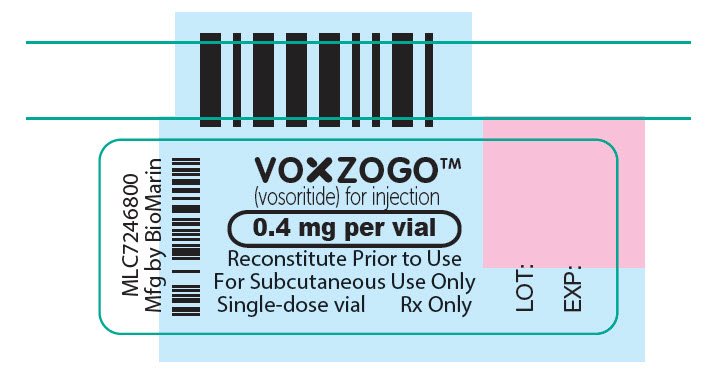
PRINCIPAL DISPLAY PANEL – 0.4 MG VIAL LABEL
VOXZOGO™
(vosoritide) for injection
0.4 mg per vial
Reconstitute Prior to Use
For Subcutaneous Use Only
Single-dose vial
Rx Only
LOT:
EXP:
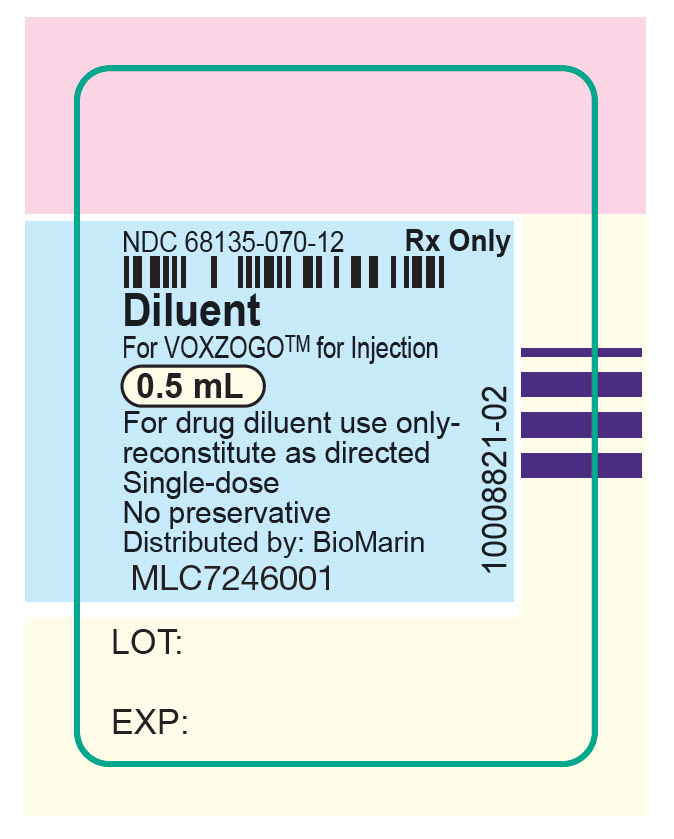
PRINCIPAL DISPLAY PANEL – 0.5 ML SYRINGE LABEL
NDC 68135-070-12
Rx Only
Diluent
For VOXZOGO™ for Injection
0.5 mL
For drug diluent use only-
reconstitute as directed
Single-dose
No preservative
Distributed by: BioMarin
MLC7246001
LOT:
EXP:
10008821-02
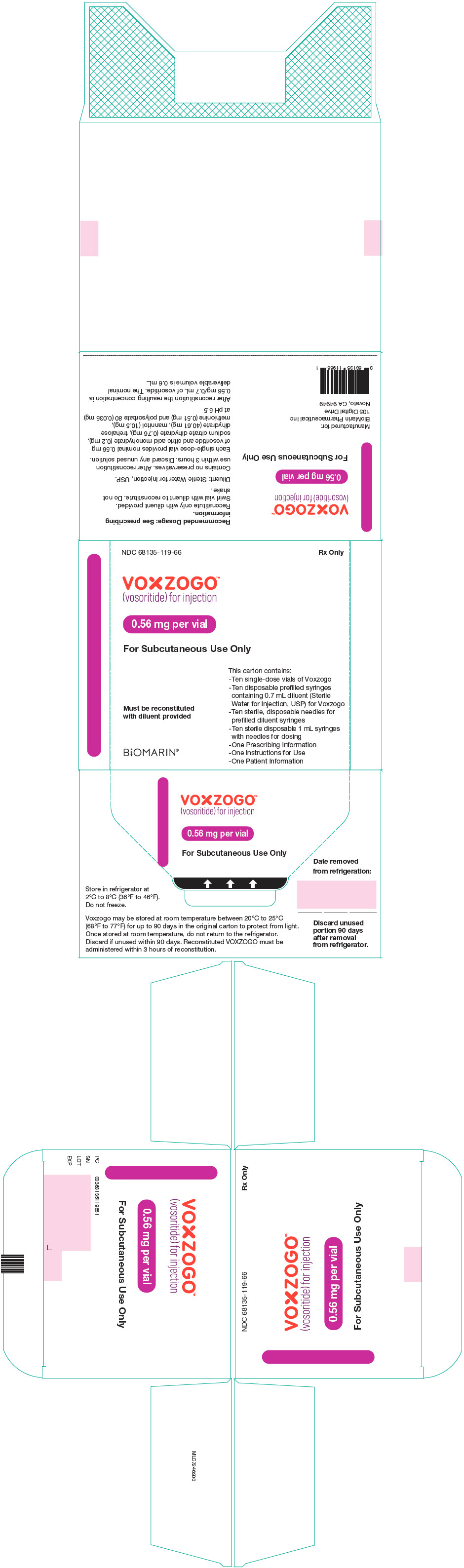
PRINCIPAL DISPLAY PANEL – KIT CARTON – 68135-119
NDC 68135-119-66
Rx Only
VOXZOGO™
(vosoritide) for injection
0.56 mg per vial
For Subcutaneous Use Only
This carton contains:
– Ten single-dose vials of Voxzogo
– Ten disposable prefilled syringes
containing 0.7 mL diluent (Sterile
Water for Injection, USP) for Voxzogo
– Ten sterile, disposable needles for
prefilled diluent syringes
– Ten sterile disposable 1 mL syringes
with needles for dosing
– One Prescribing Information
– One Instructions for Use
– One Patient Information
Must be reconstituted
with diluent provided
BiOMARIN®
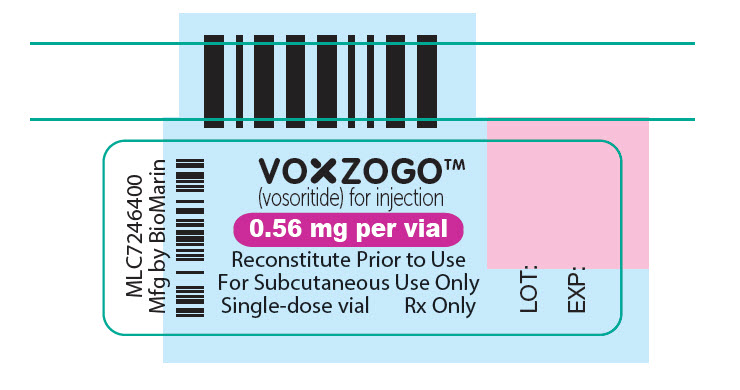
PRINCIPAL DISPLAY PANEL – 0.56 MG VIAL LABEL
VOXZOGO™
(vosoritide) for injection
0.56 mg per vial
Reconstitute Prior to Use
For Subcutaneous Use Only
Single-dose vial
Rx Only
LOT:
EXP:
PRINCIPAL DISPLAY PANEL – 0.7 ML SYRINGE LABEL
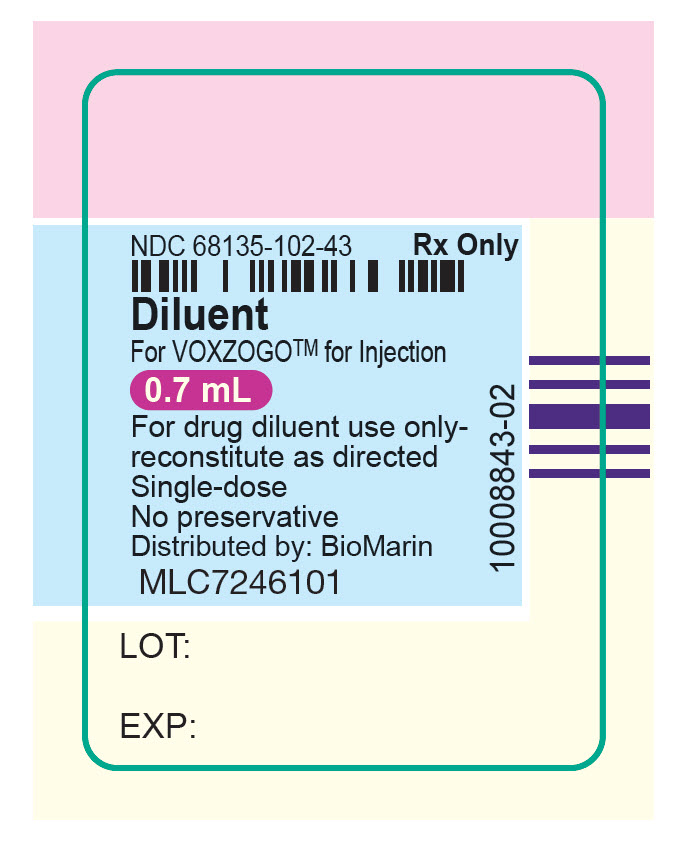
NDC 68135-102-43
Rx Only
Diluent
For VOXZOGO™ for Injection
0.7 mL
For drug diluent use only-
reconstitute as directed
Single-dose
No preservative
Distributed by: BioMarin
MLC7246001
LOT:
EXP:
10008843-02
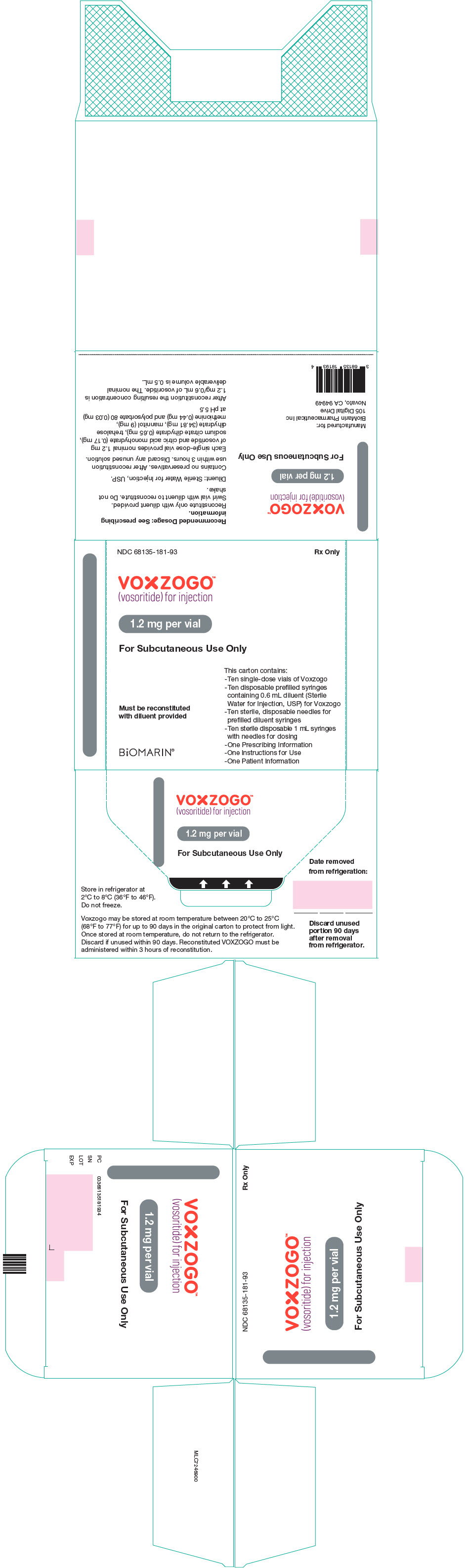
PRINCIPAL DISPLAY PANEL – KIT CARTON – 68135-181
NDC 68135-181-93
Rx Only
VOXZOGO™
(vosoritide) for injection
1.2 mg per vial
For Subcutaneous Use Only
This carton contains:
– Ten single-dose vials of Voxzogo
– Ten disposable prefilled syringes
containing 0.6 mL diluent (Sterile
Water for Injection, USP) for Voxzogo
– Ten sterile, disposable needles for
prefilled diluent syringes
– Ten sterile disposable 1 mL syringes
with needles for dosing
– One Prescribing Information
– One Instructions for Use
– One Patient Information
Must be reconstituted
with diluent provided
BiOMARIN®
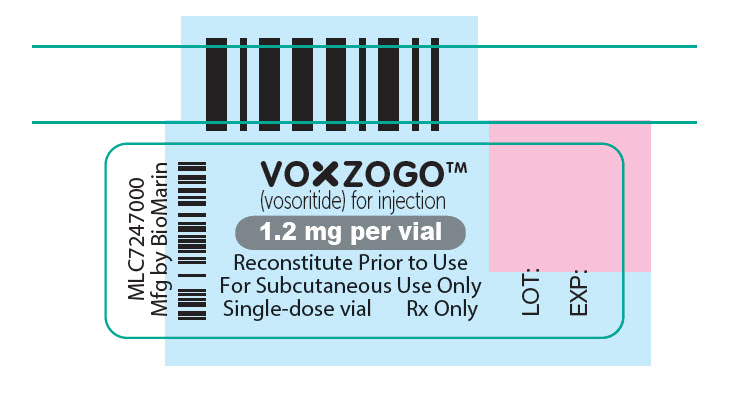
PRINCIPAL DISPLAY PANEL – 1.2 MG VIAL LABEL
VOXZOGO™
(vosoritide) for injection
1.2 mg per vial
Reconstitute Prior to Use
For Subcutaneous Use Only
Single-dose vial
Rx Only
LOT:
EXP:
PRINCIPAL DISPLAY PANEL – 0.6 ML SYRINGE LABEL
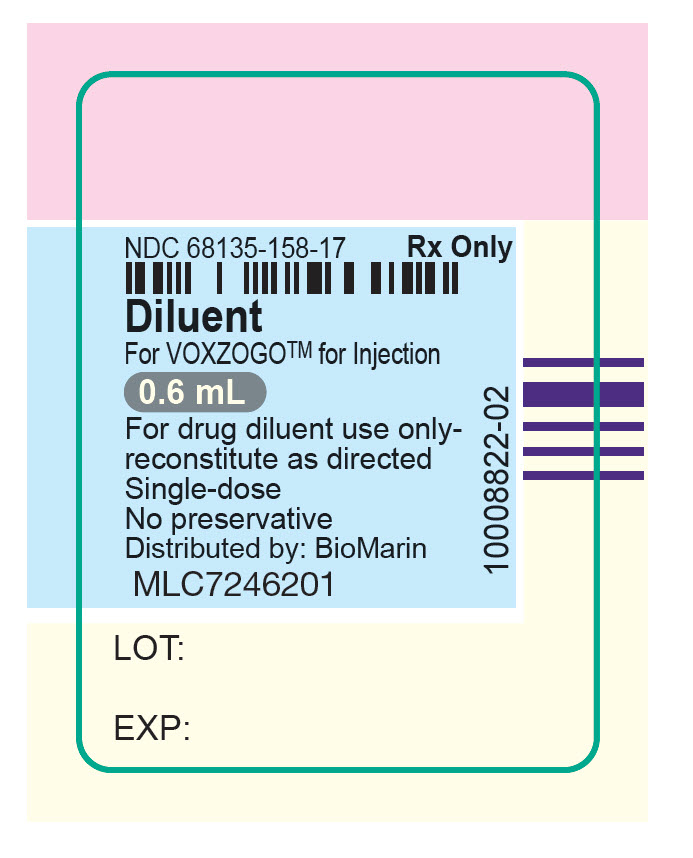
NDC 68135-158-17
Rx Only
Diluent
For VOXZOGO™ for Injection
0.6 mL
For drug diluent use only-
reconstitute as directed
Single-dose
No preservative
Distributed by: BioMarin
MLC7246201
LOT:
EXP:
10008822-02