Xaciato (Vaginal Gel)
Generic name: clindamycin phosphate
Dosage form: vaginal gel
Drug class: Vaginal anti-infectives
Medically reviewed by A Ras MD. Last updated Apri 5, 2022
What is Xaciato
Xaciato Vaginal Gel is an anti-infective medicine with an active ingredient of clindamycin phosphate.
1. INDICATION AND USAGEIndication and usage
Bacterial Vaginosis

XACIATO is indicated for the treatment of bacterial vaginosis in females 12 years and older.
2. DOSAGE AND ADMINISTRATION
The recommended dosage of XACIATO is one applicatorful (5 g of a vaginal gel containing 100 mg of clindamycin) administered once intravaginally as a single dose at any time of the day. Place the used tube with any remaining gel and used applicator in the container box and deposit it in a trash container after use.
XACIATO is not for ophthalmic, dermal, or oral use.
3. DOSAGE FORMS AND STRENGTHS
Vaginal gel: 2% clindamycin (present as clindamycin phosphate) as a clear, colorless, viscous gel in a 25 g tube. One user-filled single-dose disposable applicator delivers 5 g of gel containing 100 mg of clindamycin.
4. CONTRAINDICATIONS
Hypersensitivity
XACIATO is contraindicated in individuals with a history of hypersensitivity to clindamycin or lincomycin.
5. WARNINGS AND PRECAUTIONS
Clostridioides difficile-Associated Diarrhea (CDAD)
Clostridioides difficile-associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including clindamycin, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon which can lead to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibacterial use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents. Patients with inflammatory bowel disease, including ulcerative colitis and Crohn’s disease, have a higher risk of developing CDAD.
If CDAD is suspected or confirmed, ongoing antibacterial use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibacterial treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated
Use with Polyurethane Condoms
XACIATO is not compatible with and may weaken polyurethane condoms; therefore, their use is not recommended during treatment with XACIATO or for 7 days following treatment. During this time period, polyurethane condoms may not be reliable for preventing pregnancy or for protecting against the transmission of HIV and other sexually transmitted diseases. Latex or polyisoprene condoms should be used.
Vaginal Candida Infections
XACIATO may result in the overgrowth of Candida spp. in the vagina resulting in vulvovaginal candidiasis which may require antifungal treatment.
6. ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Clostridioides difficile-Associated Diarrhea (CDAD)
- Use with Polyurethane Condoms
- Vaginal Candida Infections
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In the placebo-controlled trial (Trial 1), 202 patients with bacterial vaginosis were treated with a single dose of XACIATO, and 103 patients were treated with a single dose of placebo gel. The median age of the patients in the trial was 35 years (range 15-59 years). The population was 56% Black or African American and 41% White. Persons of Hispanic or Latino ethnicity made up 25% of the population. A history of prior bacterial vaginosis was noted in 89% of the population.
Most Common Adverse Reactions
Adverse reactions were reported by 76/202 (38%) of patients who received XACIATO and 28/103 (27%) of patients who received placebo in Trial 1. Table 1 displays the most common adverse reactions (occurring in >2% of patients and at a higher rate in the XACIATO group than in the placebo group) in Trial 1.
|
||
|
Adverse Reaction |
XACIATO N=202 n (%) |
Placebo N=103 n (%) |
|
Vulvovaginal candidiasis |
35 (17) |
4 (4) |
|
Vulvovaginal discomfort* |
13 (6) |
5 (5) |
Other Clindamycin Formulations
XACIATO affords low peak serum levels and systemic exposure of clindamycin compared to an oral or intravenous dose of clindamycin. Data from well-controlled trials directly comparing clindamycin administered orally to clindamycin administered vaginally are not available.
The following additional adverse reactions and altered laboratory tests have been reported with the oral or parenteral use of clindamycin:
Gastrointestinal: Abdominal pain, esophagitis, nausea, Clostridioides difficile-associated diarrhea.
Hematopoietic: Transient neutropenia (leukopenia), eosinophilia, agranulocytosis, and thrombocytopenia have been reported. No direct etiologic relationship to concurrent clindamycin therapy could be made in any of these reports.
Hypersensitivity Reactions: Maculopapular rash, vesiculobullous rash, and urticaria have been observed during drug therapy. Generalized mild to moderate morbilliform-like skin rashes are the most frequently reported of all adverse reactions. Cases of erythema multiforme, some resembling Stevens-Johnson syndrome, have been associated with clindamycin. A few cases of anaphylactoid reactions have been reported.
Liver: Jaundice and abnormalities in liver function tests have been observed during clindamycin therapy.
Musculoskeletal: Cases of polyarthritis have been reported.
Renal: Acute kidney injury.
7. DRUG INTERACTIONS
Neuromuscular Blocking Agents
Systemic clindamycin has neuromuscular blocking properties that may enhance the action of other neuromuscular blocking agents. Therefore, it should be used with caution in patients receiving such agents.
8. USE IN SPECIFIC POPULATIONS
Pregnancy
Risk Summary
Other clindamycin vaginal products have been used to treat pregnant women during the second and third trimester. XACIATO has not been studied in pregnant women. However, based on the low systemic absorption of XACIATO following the intravaginal route of administration in nonpregnant women, maternal use is not likely to result in significant fetal exposure to the drug. Available data from published observational studies, based on first trimester exposure to oral and IV clindamycin, did not identify consistent increases in the rate of major birth defects.
Available data from published observational studies and randomized controlled trials, based on second and third trimester exposure to oral and IV clindamycin, did not identify an increased risk of miscarriage or other adverse maternal or fetal outcomes. Most of the reported exposures to clindamycin occurred during the second and third trimesters of the pregnancy.
In animal reproduction studies, no adverse developmental outcomes were observed when XACIATO was vaginally administered to pregnant rats and rabbits during organogenesis at doses approximately equivalent to the recommended human dose. No evidence of any adverse developmental outcomes was observed when oral or subcutaneous doses of clindamycin were administered to pregnant rats and mice during organogenesis at doses 9 to 58 times the recommended human dose based on body surface area comparison (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
Reproduction studies performed during organogenesis in pregnant rats (gestational days 6-17) and rabbits (gestational days 7-19) administered vaginal XACIATO at 0.1 g and 1 g/day (2 mg and 20 mg clindamycin phosphate/day) showed no evidence of developmental toxicity. These doses are approximately equivalent to the applied recommended clinical dose based on g/cm2 vaginal surface area and body surface area (BSA) comparisons.
Reproduction studies performed during organogenesis (gestational days 6-15) in pregnant rats and mice that were administered oral doses of clindamycin up to 600 mg/kg/day (58 and 29 times, respectively, the recommended human dose based on a body surface area comparison) or subcutaneous doses of clindamycin up to 250 mg/kg/day (24- and 12-times, respectively, the MRHD based on BSA comparisons) revealed no evidence of teratogenicity.
Vaginal administration of XACIATO to pregnant/lactating female rats during a pre-and postnatal development (gestation day 6 through gestation day 21) study at 2 mg clindamycin phosphate/day had no adverse effects on dams or their offspring.
Lactation
Risk Summary
Systemic absorption following intravaginal administration of clindamycin is low; therefore, transfer of clindamycin into breastmilk is likely to be low and adverse effects on the breastfed infant are not expected. There are no data on the effect of clindamycin on milk production.
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for clindamycin and any potential adverse effects on the breastfed child from clindamycin or from the underlying maternal condition.
Males and Females of Reproductive Potential
Contraception
XACIATO is not compatible with and may weaken polyurethane condoms; therefore, use of polyurethane condoms is not recommended during and for 7 days following treatment with XACIATO. Advise patients to use latex or polyisoprene condoms for contraception during and for 7 days following treatment with XACIATO.
Pediatric Use
The safety and effectiveness of XACIATO have been established in females aged 12 years and older for the treatment of bacterial vaginosis. Use of XACIATO for this indication is supported by the extrapolation of clinical trial data from adequate and well controlled clinical studies in adult women. The safety and effectiveness of XACIATO have not been established in pediatric patients younger than 12 years of age for the treatment of bacterial vaginosis.
Geriatric Use
Clinical studies with XACIATO did not include any subjects 65 years of age or older to determine whether they respond differently than younger subjects.
11. DESCRIPTION
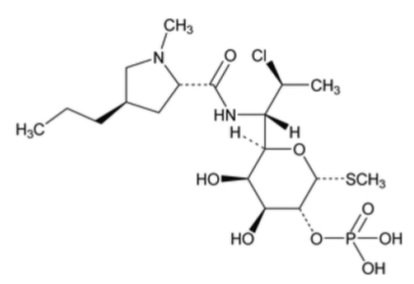
Xaciato Vaginal Gel contains clindamycin phosphate, a lincosamide antibacterial. The chemical name for clindamycin phosphate is methyl 7-chloro- 6,7,8-trideoxy-6-(1-methyl- trans-4-propyl-L-2-pyrrolidinecarboxamido)-1-thio-L- threo-(alpha)-D-galacto- octopyranoside 2-(dihydrogenphosphate). It has a molecular weight of 504.95, and the molecular formula is C18H34ClN2O8PS. The structural formula is represented below:
XACIATO is a clear, colorless, viscous gel, which contains clindamycin at a concentration of 2%. A single-dose user-filled disposable applicator delivers 5 g of vaginal gel containing 100 mg of clindamycin (present as 119 mg of clindamycin phosphate). The gel also contains benzyl alcohol, citric acid monohydrate, poloxamer 407, purified water, sodium citrate dihydrate, and xanthan gum.¶
12. CLINICAL PHARMACOLOGY
Mechanism of Action
Clindamycin is an antibacterial drug
Pharmacokinetics
Clindamycin phosphate is a water-soluble ester prodrug of clindamycin. Biologically inactive clindamycin phosphate is converted to active clindamycin. Following a single intravaginal dose of 100 mg of XACIATO administered to 21 healthy female subjects, the arithmetic mean (range) peak plasma concentration was 69.2 ng/mL (3.8 to 236 ng/mL). The median (range) tmax occurred at 6 hours (4 to 96 hours). The mean Cmax of clindamycin for XACIATO was approximately 0.5% of that observed after the IV infusion of Clindamycin Phosphate in 0.9% Sodium Chloride, 900 mg clindamycin, every 8 hours.
Microbiology
Mechanism of Action
Clindamycin inhibits bacterial protein synthesis by binding to the 23S RNA of the 50S subunit of the ribosome. Clindamycin is predominantly bacteriostatic. Although clindamycin phosphate is inactive in vitro, rapid in vivo hydrolysis converts it to active clindamycin.
Resistance
Resistance to clindamycin is most often caused by modification of the target site on the ribosome, usually by chemical modification of RNA bases by point mutations in RNA or occasionally in proteins. Cross resistance has been demonstrated between lincosamides, macrolides and streptogramins B in some organisms. Cross resistance has been demonstrated between clindamycin and lincomycin.
Antibacterial Activity
Culture and sensitivity testing of bacteria are not routinely performed to establish the diagnosis of bacterial vaginosis. Standard methodology for the susceptibility testing of the potential bacterial pathogens, Gardnerella vaginalis, Mobiluncus spp., or Mycoplasma hominis, has not been defined.
The following in vitro data are available, but their clinical significance is unknown. Clindamycin is active in vitro against most isolates of the following organisms reported to be associated with bacterial vaginosis:
Bacteroides spp.
Gardnerella vaginalis
Mobiluncus spp.
Mycoplasmahominis
Peptostreptococcus spp.
13. NONCLINICAL TOXICOLOGY
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Long term studies in animals to evaluate carcinogenic potential have not been performed with XACIATO, or the active ingredient, clindamycin phosphate.
Mutagenesis
The genotoxic potential of clindamycin has been evaluated in a rat micronucleus test and an Ames Salmonella reversion test. Both tests were negative.
Impairment of Fertility
Fertility studies in rats treated vaginally with 2 mg/day clindamycin phosphate (0.1g/day XACIATO) or orally with 300 mg/kg/day (doses that are approximately equivalent to or 29-times the MRHD based on BSA comparisons, respectively) revealed no effects on fertility or mating ability.
14. CLINICAL STUDIES
The efficacy of XACIATO as a treatment of bacterial vaginosis (BV) in females 12 years of age and older was demonstrated in a randomized, double-blind, placebo-controlled clinical study (Trial 1, NCT04370548). A single dose of XACIATO (clindamycin phosphate vaginal gel, 2%) was compared to a single dose of placebo vaginal gel (hydroxyethylcellulose [HEC] Universal Placebo Gel) for the treatment of BV. Patients were evaluated at 3 timepoints: a Day 1 screening/randomization visit, a Day 7 to 14 Interim Assessment visit, and a Day 21 to 30 Test of Cure visit. The total study duration was up to approximately 1 month for each individual patient.
To be eligible, patients had to have a clinical diagnosis of BV defined as an off-white (milky or gray), thin, homogeneous discharge with minimal or absent pruritus and inflammation of the vulva and vagina, clue cells > 20% of the total epithelial cells on microscopic examination of the saline wet mount, vaginal secretion pH of > 4.5, and a fishy odor of the vaginal discharge with the addition of a drop of 10% KOH (i.e., a positive whiff test).
The 307 patients were randomized in a 2:1 ratio, with 204 in the XACIATO group and 103 in the placebo group. The modified Intent-To-Treat (mITT) Population excluded women with a positive test result for other concomitant vaginal or cervical infections at baseline, including a positive vaginal culture for Candida spp. or who had a baseline Nugent score of < 7.
Clinical Cure was defined as resolution of the abnormal vaginal discharge associated with BV, a negative 10% KOH whiff test, and clue cells < 20% of the total epithelial cells in the saline wet mount. Bacteriological Cure was defined as a Nugent score < 4. Therapeutic Cure was defined as the presence of both a Clinical Cure and Bacteriological Cure.
In the mITT population, a statistically significantly greater percentage of patients experienced Clinical Cure, Bacteriological Cure, and Therapeutic Cure at the Test of Cure (Day 21-30) visit in the XACIATO arm compared to placebo (Table 2). Statistically significant results for the endpoints were also achieved at the Interim Assessment visit (Day 7-14).
|
Interim Assessment visit (day 7-14) |
Test of Cure visit (day 21-30) |
|||||
|
Parameter |
XACIATO |
Placebo |
Treatment |
XACIATO |
Placebo |
Treatment Difference (%) |
|
Clinical Cure |
93 (76.2) |
14 (23.7) |
52.5 (38.0, 67.0) |
86 (70.5) |
21 (35.6) |
34.9 (19.0, 50.8) |
|
Bacteriological Cure |
50 (41.0) |
2 ( 3.4) |
37.6 (26.5, 48.7) |
53 (43.4) |
3 ( 5.1) |
38.4 (26.7, 50.1) |
|
Therapeutic Cure |
43 (35.2) |
0 |
35.2 (25.5, 45.0) |
45 (36.9) |
3 ( 5.1) |
31.8 (20.3, 43.3) |
All P-values for differences between treatment groups were <0.001 (from Cochran-Mantel-Haenszel test with strata of study site and race – African American or all other races).
The percentage of patients with Clinical Cure at the Test of Cure visit was also significantly higher in the XACIATO group compared to the placebo group among the subsets of patients defined by prior episodes of bacterial vaginosis (≤ 3 episodes and >3 episodes in the previous 12 months) at 71.3% (72/101) for XACIATO and 39.1% (18/46) placebo, and 70.0% (14/20) for XACIATO and 23.1% (3/13) placebo, respectively.
16. HOW SUPPLIED/STORAGE AND HANDLING
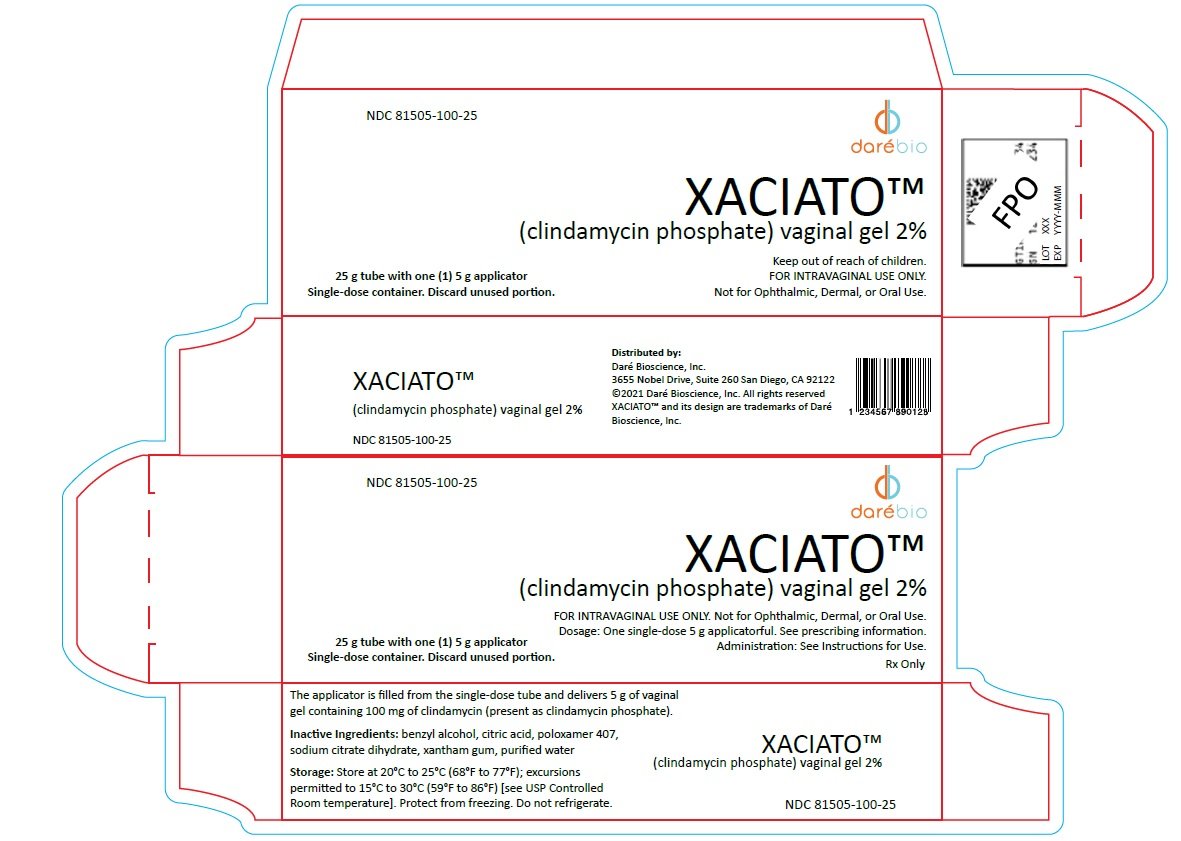
Xaciato Vaginal Gel, 2% is a clear, colorless, viscous gel supplied in a carton containing one 25-gram tube of vaginal gel and one vaginal applicator. One single-dose, user-filled disposable applicator delivers 5 g of gel containing 100 mg of clindamycin. NDC number 81505-100-25.
Store at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [See USP Controlled Room temperature].
17. PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Instructions for Use).
Vaginal Intercourse and Use with Vaginal Products
Instruct the patients not to engage in vaginal intercourse or use other vaginal products (such as tampons or douches) during treatment with XACIATO and for 3 days after using XACIATO.
Use with Polyurethane Condoms
Advise the patient that XACIATO may weaken polyurethane condoms. Therefore, use of polyurethane condoms concurrently or for 7 days following treatment with XACIATO is not recommended. During this time period polyurethane condoms may not be reliable for preventing pregnancy or for protecting against transmission of HIV and other sexually transmitted diseases. Latex or polyisoprene condoms should be used.
Vaginal Candida Infections
Inform the patient that vaginal yeast infections can occur following use of XACIATO and may require treatment with an antifungal drug.
Manufactured for:
Daré Bioscience, Inc.
3655 Nobel Drive, Suite 260
San Diego, CA 92122
INSTRUCTIONS FOR USE
XACIATO™ [zah-she-AH-toe]
(clindamycin phosphate vaginal gel 2%)
For vaginal use only. Do not use in the eyes or mouth, or on your skin.
Read these Instructions for Use before you use XACIATO™ and each time you get a refill as there may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or treatment.
Important Information
- Use XACIATO exactly as your healthcare provider tells you to use it.
- Use 1 filled applicator of XACIATO gel in your vagina.
- Do not use any medicine from the tube or applicator more than 1 time. The XACIATO gel is for 1 time (single-use) only.
- Do not have vaginal sex or use vaginal products (such as tampons or douches) during treatment with XACIATO gel and for 3 days after using XACIATO gel.
- Do not use polyurethane condoms during treatment with XACIATO gel and for 7 days after using XACIATO gel. XACIATO gel may weaken polyurethane condoms. Latex or polyisoprene condoms should be used.
- If you have questions or if your vaginal symptoms do not go away, contact your healthcare provider.
How do I store XACIATO?
- Store XACIATO gel at room temperature between 68° to 77°F (20° to 25°C).
- Do not store in the refrigerator or freezer.
Store XACIATO gel and all medicines out of the reach of children.
|
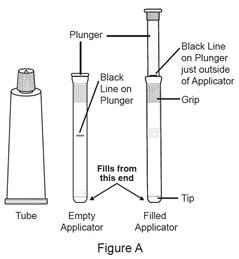
You will need the following included supplies (see Figure A):
|
 |
| Step 1. Remove the applicator and tube from the container box. | |
|
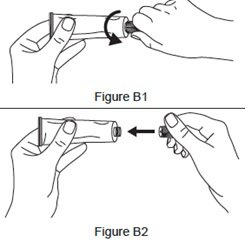
Step 2. Open the tube.
|
 |
|
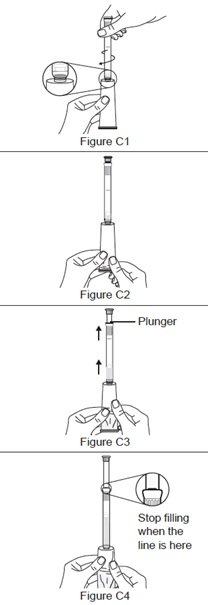
Step 3. Fill the applicator.
|
 |
|
Step 4. Prepare to insert the filled applicator.
|
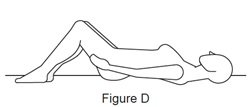 |
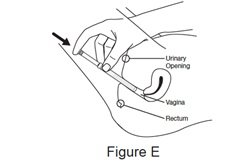
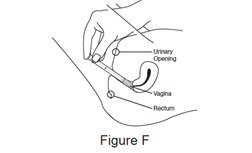
Step 5. Insert the filled applicator.
|
 |
Step 6. Push the plunger.
|
 |
| Step 7. Remove the empty applicator from your vagina. | |
| Step 8. Place the used applicator and tube in the container box and throw away (dispose of) in your household trash. |
This Instructions for Use has been approved by the U.S. Food and Drug Administration. 12/2021
Carton
Label