Xofluza
Generic name: baloxavir marboxil
Drug class: Miscellaneous antivirals
Medically reviewed by A Ras MD.
What is Xofluza?
Xofluza is a prescription medicine used to treat the flu (influenza) in people 12 years of age and older who have had flu symptoms for no more than 48 hours.
It is also used to prevent the flu in people 12 years of age and older following contact with a person who has the flu.
It is not known if Xofluza is safe and effective in children less than 12 years of age.
Xofluza does not treat or prevent illness that is caused by infections other than the influenza virus.
Xofluza does not prevent bacterial infections that may happen with the flu.
Description
XOFLUZA (baloxavir marboxil) is an antiviral PA endonuclease inhibitor.
The active component of XOFLUZA is baloxavir marboxil. The chemical name of baloxavir marboxil is ({(12aR)-12-[(11S)-7,8-Difluoro-6,11-dihydrodibenzo[b,e]thiepin-11-yl]-6,8-dioxo-3,4,6,8,12,12a-hexahydro-1H-[1,4]oxazino[3,4-c]pyrido[2,1-f][1,2,4]triazin-7-yl}oxy)methyl methyl carbonate. The empirical formula of baloxavir marboxil is C27H23F2N3O7S, and the chemical structure is shown below.
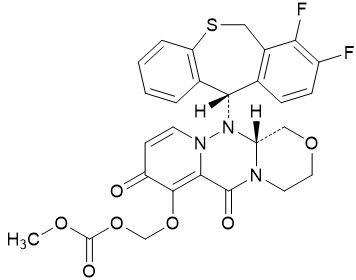
Baloxavir marboxil has a molecular mass of 571.55 grams per mole and a partition coefficient (log P) of 2.26. It is freely soluble in dimethylsulfoxide, soluble in acetonitrile, slightly soluble in methanol and ethanol, and practically insoluble in water.
XOFLUZA is supplied as tablets and as granules for oral suspension:
XOFLUZA tablets are white to light yellow, film-coated tablets for oral administration. The 40 mg film-coated tablet contains 40 mg of baloxavir marboxil and the 80 mg film-coated tablet contains 80 mg of baloxavir marboxil. The inactive ingredients of XOFLUZA tablets are: croscarmellose sodium, hypromellose, lactose monohydrate, microcrystalline cellulose, povidone, sodium stearyl fumarate, talc, and titanium dioxide.
XOFLUZA for oral suspension is supplied as white to light yellow granules in an amber glass bottle. Each bottle contains 40 mg (nominal) of baloxavir marboxil. The granules must be constituted with 20 mL of drinking water or sterile water to yield a 2 mg/mL suspension with strawberry flavor. The inactive ingredients are: colloidal silicon dioxide, hypromellose, maltitol, mannitol, povidone K25, sodium chloride, strawberry flavor, sucralose and talc.
Who should not take Xofluza?
Do not take Xofluza if you are allergic to baloxavir marboxil or any of the ingredients in Xofluza.
See the end of this guide for a complete list of ingredients in Xofluza.
What should I tell my healthcare provider before taking Xofluza?
Before you take Xofluza, tell your healthcare provider about all of your medical conditions, including if you:
- are pregnant or plan to become pregnant. It is not known if Xofluza can harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if Xofluza passes into your breast milk.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Talk to your healthcare provider before you receive a live flu vaccine after taking Xofluza.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I take Xofluza?
- Take Xofluza exactly as directed by your healthcare provider or pharmacist.
- Your healthcare provider will either prescribe:
- Xofluza tablets that you will take at the same time as a single dose, or
- Xofluza oral suspension provided with a measuring device (an oral syringe or measuring cup) to be given as a single dose.
- Xofluza may be given through a feeding tube. Follow your healthcare provider’s instructions for giving Xofluza through a feeding tube.
- Take Xofluza with or without food.
- Do not take Xofluza with dairy products, calcium-fortified beverages, laxatives, antacids or oral supplements containing iron, zinc, selenium, calcium or magnesium.
- If you take too much Xofluza, go to the nearest emergency room right away.
If you are taking Xofluza for oral suspension:
- The pharmacist will mix Xofluza for oral suspension before it is given to you. If Xofluza is not given to you as a liquid or measuring device was not provided, contact your pharmacist.
- Take Xofluza before the expiration time and date written by the pharmacist on the bottle label. Do not take Xofluza if the expiration time and date have passed. Throw away (discard) the bottle and contact your healthcare provider.
- The total prescribed dose of Xofluza for oral suspension may require more than one bottle of Xofluza.
Giving a dose of Xofluza for oral suspension:
- Step 1. Swirl the Xofluza for oral suspension bottle well before each use. Do not shake.
- Step 2. Open the bottle by pushing downward on the child resistant bottle cap and twisting it in the direction of the arrow.
- Step 3. Measure the oral suspension with the measuring device provided by the pharmacist to be sure you give the prescribed dose.
- Step 4. The patient should sit in an upright position when taking Xofluza.
- Do not give Xofluza while the patient is lying down.
- Do not mix Xofluza with soft food or another liquid to give the medicine.
- Step 5. Give the full contents of the measuring device. You may need more than one bottle for your prescribed dose.
- Step 6. Close the bottle. Throw away any remaining oral suspension and the measuring device.
What are the possible side effects of Xofluza?
Xofluza may cause serious side effects, including:
- Allergic reactions. Get emergency medical help right away if you develop any of these signs and symptoms of an allergic reaction:
- trouble breathing
- skin rash, hives or blisters
- swelling of your face, throat or mouth
- dizziness or lightheadedness
The most common side effects of Xofluza in adults and adolescents include:
- diarrhea
- bronchitis
- sinusitis
- headache
- nausea
Xofluza is not effective in treating or preventing infections other than influenza. Other kinds of infections can appear like flu or occur along with flu and may need different kinds of treatment.
Tell your healthcare provider if you feel worse or develop new symptoms during or after treatment with Xofluza or if your flu symptoms do not start to get better.
These are not all the possible side effects of Xofluza.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Xofluza
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Xofluza for a condition for which it was not prescribed. Do not give Xofluza to other people, even if they have the same symptoms that you have. It may harm them. You can ask for information about Xofluza that is written for health professionals.
How should I store Xofluza?
Xofluza tablets:
- Store Xofluza tablets at room temperature between 68°F to 77°F (20°C to 25°C).
- Store Xofluza tablets in the blister package that they come in.
Xofluza for oral suspension:
- Store Xofluza for oral suspension at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep Xofluza for oral suspension in the original container. Use by the expiration time and date written on bottle label.
- Throw away any Xofluza for oral suspension not used by the time and date on the bottle label.
Keep Xofluza and all medicines out of the reach of children.
What are the ingredients in Xofluza?
Active ingredient: baloxavir marboxil
Inactive ingredients:
Tablets: croscarmellose sodium, hypromellose, lactose monohydrate, microcrystalline cellulose, povidone, sodium stearyl fumarate, talc, and titanium dioxide.
Oral suspension: colloidal silicon dioxide, hypromellose, maltitol, mannitol, povidone K25, sodium chloride, strawberry flavor, sucralose and talc.
Label
PRINCIPAL DISPLAY PANEL – 40 MG/20 ML BOTTLE CARTON
- NDC 50242-583-01
- Xofluza®
(baloxavir marboxil)
for oral suspension - 40 mg/20 mL (2 mg/mL)
- Each mL contains
2 mg baloxavir marboxil
after constitution. - Take volume prescribed as a
single one-time dose.
Discard unused portion. - Use the appropriate
measuring device for your
total prescribed dose. - Take before the suspension
expires (see bottle label). - No preservative.
- 20 mL total usable volume
after constitution - Rx only
Genentech - 10221949

SRC: NLM .