Zepatier
Generic name: elbasvir and grazoprevir
Drug class: Antiviral combinations
Medically reviewed by A Ras MD.
What is Zepatier?
Zepatier is a prescription medicine used with or without ribavirin to treat chronic (long-lasting) hepatitis C virus (HCV) genotypes 1 or 4 infection in adults.
It is not known if Zepatier is safe or effective in children under 18 years old, people awaiting a liver transplant, or people who have had a liver transplant.
Description
ZEPATIER is a fixed-dose combination tablet containing elbasvir and grazoprevir for oral administration.
Elbasvir is an HCV NS5A inhibitor, and grazoprevir is an HCV NS3/4A protease inhibitor.
Each tablet contains 50 mg elbasvir and 100 mg grazoprevir. The tablets include the following inactive ingredients: colloidal silicon dioxide, copovidone, croscarmellose sodium, hypromellose, lactose monohydrate, magnesium stearate, mannitol, microcrystalline cellulose, sodium chloride, sodium lauryl sulfate, and vitamin E polyethylene glycol succinate. The tablets are film-coated with a coating material containing the following inactive ingredients: carnauba wax, ferrosoferric oxide, hypromellose, iron oxide red, iron oxide yellow, lactose monohydrate, titanium dioxide, and triacetin.
Elbasvir:
The IUPAC name for elbasvir is Dimethyl N,N’-([(6S)-6-phenylindolo[1,2-c][1,3]benzoxazine-3,10-diyl]bis{1H-imidazole-5,2-diyl-(2S)-pyrrolidine-2,1-diyl[(2S)-3-methyl-1-oxobutane-1,2-diyl]})dicarbamate.
It has a molecular formula of C49H55N9O7 and a molecular weight of 882.02. It has the following structural formula:
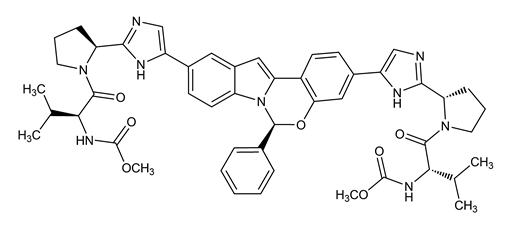
Elbasvir is practically insoluble in water (less than 0.1 mg per mL) and very slightly soluble in ethanol (0.2 mg per mL), but is very soluble in ethyl acetate and acetone.
Grazoprevir:
The IUPAC name for grazoprevir is (1aR,5S,8S,10R,22aR)-N-[(1R,2S)-1-[(Cyclopropylsulfonamido)carbonyl]-2-ethenylcyclopropyl]-14-methoxy-5-(2-methylpropan-2-yl)-3,6-dioxo-1,1a,3,4,5,6,9,10,18,19,20,21,22,22a-tetradecahydro-8H-7,10-methanocyclopropa[18,19][1,10,3,6]dioxadiazacyclononadecino[11,12-b]quinoxaline-8-carboxamide.
It has a molecular formula of C38H50N6O9S and a molecular weight of 766.90. It has the following structural formula:
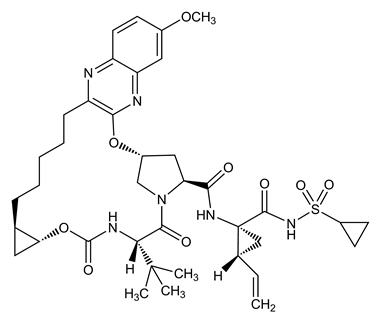
Grazoprevir is practically insoluble in water (less than 0.1 mg per mL) but is freely soluble in ethanol and some organic solvents (e.g., acetone, tetrahydrofuran and N,N-dimethylformamide).
Mechanism of Action
ZEPATIER is a fixed-dose combination of elbasvir and grazoprevir which are direct-acting antiviral agents against the hepatitis C virus
What is the most important information I should know about Zepatier?
Zepatier can cause serious side effects, including:
- Hepatitis B virus reactivation: Before starting treatment with Zepatier, your healthcare provider will do blood tests to check for hepatitis B virus infection. If you have ever had hepatitis B virus infection, the hepatitis B virus could become active again during or after treatment of hepatitis C virus infection with Zepatier. Hepatitis B virus becoming active again (called reactivation) may cause serious liver problems including liver failure and death. Your healthcare provider will monitor you if you are at risk for hepatitis B virus reactivation during treatment and after you stop taking Zepatier.
For more information about side effects, see the section “What are the possible side effects of Zepatier?”
Other information you need to know about Zepatier
- Before you take this medicine, be sure you understand what it is for and how to take it safely.
- Keep this information.
- If you have questions about this medicine, ask your healthcare provider or pharmacist.
- Every time you get a refill, look at the Patient Information. There may be new information.
- Your healthcare provider may prescribe Zepatier with a medicine called ribavirin. Ribavirin is also known as Rebetol, Copegus, Ribasphere, and Moderiba. If you take Zepatier and ribavirin, be sure you read the Medication Guide for ribavirin.
Who should not take Zepatier?
Do not take Zepatier if you have certain liver problems.
What should I tell my healthcare provider before taking Zepatier?
Before taking Zepatier, tell your healthcare provider about all of your medical conditions, including if you:
- have ever had hepatitis B virus infection
- have liver problems other than hepatitis C
- have ever taken any medicine for hepatitis C
- have HIV
- have had or are waiting for a liver transplant
- are pregnant or trying to get pregnant. Zepatier has not been studied in pregnant women. We do not know if Zepatier will harm your baby while you are pregnant.
- Males and females who take Zepatier and ribavirin should also read the ribavirin Medication Guide for important pregnancy, contraception, and infertility information.
- are breastfeeding or plan to breastfeed. We do not know if Zepatier gets in your breast milk and gets passed to your baby.
- Talk to your healthcare provider about the best way to feed your baby during treatment with Zepatier.
Are you taking other medicines?
Tell your healthcare provider about all of the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Zepatier may affect the way other medicines work, and other medicines may affect how Zepatier works. Some medicines cannot be taken with Zepatier. Your healthcare provider can tell you if it is safe to take Zepatier with other medicines.
- Know the medicines you take. Keep a list of your medicines and show it to your healthcare provider and pharmacist when you get a new medicine.
- You can ask your healthcare provider or pharmacist for a list of medicines that interact with this medicine.
- Do not start taking a new medicine without telling your healthcare provider.
How should I take Zepatier?
- Take 1 Zepatier tablet at the same time every day.
- Zepatier comes in a blister package of individually-packaged tablets. Keep the tablets in this package until you are ready to take your dose.
- Take Zepatier exactly as your healthcare provider tells you to take it.
- Take Zepatier with or without food.
- Do not stop taking Zepatier without first talking with your healthcare provider.
- If you take more than your prescribed dose, call your healthcare provider right away.
What if I forget to take Zepatier?
- Do not take two doses of Zepatier at the same time to make up for a missed dose.
- If you are not sure what to do, call your healthcare provider or pharmacist. It is important that you do not miss or skip doses of Zepatier during treatment.
What are the possible side effects of Zepatier?
Zepatier can cause serious side effects, including:
Hepatitis B virus reactivation. See “What is the most important information I should know about Zepatier?”
Signs of liver problems. Zepatier may cause increases in your liver-related blood tests. This could be a sign of serious liver problems. Your healthcare provider will do blood tests to check your liver before and during treatment with Zepatier. Tell your healthcare provider right away if you get any of the following symptoms or if they get worse during treatment with Zepatier:
- loss of appetite
- nausea and vomiting
- feeling tired or weak
- confusion
- sleepiness
- vomiting of blood
- yellowing of your skin or eyes
- color changes in your stool or urine
- bleeding or bruising more easily than normal
- diarrhea
- swelling of the stomach area (abdomen) or pain in the upper right side of the stomach area
Common side effects of Zepatier when used without ribavirin include:
- feeling tired
- headache
- nausea
- trouble sleeping
- diarrhea
Common side effects of Zepatier when used with ribavirin include:
- low red blood cell counts (anemia)
- headache
- feeling tired
- shortness of breath
- rash or itching
- feeling irritable
- stomach pain
- depression
- joint pain
If you have any side effect that bothers you or that does not go away, tell your healthcare provider.
There may be other side effects to Zepatier that are not listed.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. For more information or medical advice call your doctor.
General information about the safe and effective use of Zepatier
- Medicines are sometimes prescribed for purposes other than those listed in the patient information. Do not use Zepatier for a condition for which it was not prescribed. Do not give Zepatier to other people, even if they have the same condition. It may harm them.
- If you would like more information, talk with your healthcare provider or pharmacist. You can ask them for information about Zepatier that was written for health professionals.
- For more information, call Merck, the company that makes Zepatier, at 1-877-888-4231 or go to www.ZEPATIER.com.
How should I store Zepatier?
- Keep Zepatier in its original packaging (blister package) until you are ready to take it. Do not take the tablets out of the original blister package to store in another container such as a pill box. This is important because the tablets are sensitive to moisture. The package is designed to protect them.
- Keep Zepatier at room temperature.
- Keep Zepatier and all medicines out of the reach of children.
What are the ingredients in Zepatier?
Active ingredients are: elbasvir and grazoprevir.
Inactive ingredients are: colloidal silicon dioxide, copovidone, croscarmellose sodium, hypromellose, lactose monohydrate, magnesium stearate, mannitol, microcrystalline cellulose, sodium chloride, sodium lauryl sulfate, and vitamin E polyethylene glycol succinate.
The tablets are film-coated with a coating material containing the following inactive ingredients: carnauba wax, ferrosoferric oxide, hypromellose, iron oxide red, iron oxide yellow, lactose monohydrate, titanium dioxide, and triacetin.
Label
PRINCIPAL DISPLAY PANEL – 50 MG/100 MG TABLET DOSE PACK CARTON
- NDC 0006-3074-02
- Zepatier®
(elbasvir and grazoprevir) tablets - 50 mg/100 mg
- Rx only
- 28 Tablets
- This carton contains a total of 28 tablets
packaged within 2 dose packs.
Each dose pack contains 14 blister units
with one tablet per blister unit.


SRC: NLM .