Zepzelca
Generic name: LURBINECTEDIN .5mg in 1mL
Dosage form: injection, powder, lyophilized, for solution
Drug class: Alkylating agents
Medically reviewed by A Ras MD.
What is Zepzelca?
Zepzelca is a prescription medicine that is used to treat adults with a kind of lung cancer called small cell lung cancer (SCLC).
Zepzelca may be used when your lung cancer has spread to other parts of the body (metastatic), and you have received treatment with chemotherapy that contains platinum, and it did not work or is no longer working.
It is not known if Zepzelca is safe and effective in children.
Description
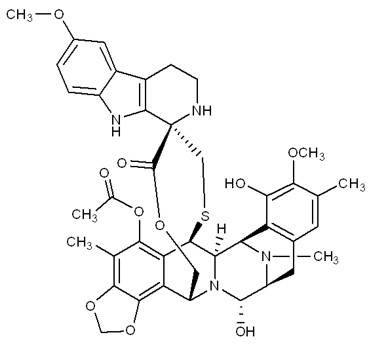
ZEPZELCA is an alkylating drug. The chemical name of ZEPZELCA (lurbinectedin) is (1’R,6R,6aR,7R,13S,14S,16R)-8,14-dihydroxy-6’,9-dimethoxy-4,10,23-trimethyl-19-oxo-2’,3’,4’,6,7,9’,12,13,14,16-decahydro-6aH-spiro[7,13-azano-6,16-(epithiopropanooxymethano) [1,3]dioxolo[7,8]isoquinolino[3,2-b][3]benzazocine-20,1’-pyrido[3,4-b]indol]-5-yl acetate.
The molecular formula is C41H44N4O10S. The molecular weight is 784.87g/mol, and the chemical structure is:
ZEPZELCA for injection 4 mg is supplied as a lyophilized powder in a single-dose vial for reconstitution for intravenous use. The ZEPZELCA lyophilized formulation is comprised of 4 mg lurbinectedin, sucrose (800 mg), lactic acid (22.1 mg), and sodium hydroxide (5.1 mg). Before use, the lyophilizate is reconstituted by addition of 8 mL Sterile Water for Injection USP, yielding a solution containing 0.5 mg/mL lurbinectedin (the calculated concentration is 0.47 mg/mL based on the final volume of 8.5 mL).
Mechanism of Action
Lurbinectedin is an alkylating drug that binds guanine residues in the minor groove of DNA, forming adducts and resulting in a bending of the DNA helix towards the major groove. Adduct formation triggers a cascade of events that can affect the subsequent activity of DNA binding proteins, including some transcription factors, and DNA repair pathways, resulting in perturbation of the cell cycle and eventual cell death.
Lurbinectedin inhibited human monocyte activity in vitro and reduced macrophage infiltration in implanted tumors in mice.
What should I tell my healthcare provider before using Zepzelca?
Before receiving Zepzelca, tell your healthcare provider about all of your medication conditions, including if you:
- have liver or kidney problems.
- are pregnant or plan to become pregnant. Zepzelca can harm your unborn baby.
- Females who are able to become pregnant:
- Your healthcare provider should do a pregnancy test before you start treatment with Zepzelca.
- You should use effective birth control (contraception) during treatment with and for 6 months after your final dose of Zepzelca.
- Tell your healthcare provider right away if you become pregnant or think that you are pregnant during treatment with Zepzelca.
Males with female partners who are able to become pregnant should use effective birth control during treatment with and for 4 months after your final dose of Zepzelca.
- are breastfeeding or plan to breastfeed. It is not known if Zepzelca passes into your breastmilk. Do not breastfeed during treatment with Zepzelca and for 2 weeks after your final dose of Zepzelca. Talk to your healthcare provider about the best way to feed your baby during treatment with Zepzelca.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Certain other medicines may affect how Zepzelca works. Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I use Zepzelca?
- Zepzelca is given by an intravenous (IV) infusion into a vein over 60 minutes.
- Zepzelca is usually given every 21 days.
- Before each treatment with Zepzelca, you may receive medicines to help prevent nausea and vomiting or make it less severe.
- Your healthcare provider will decide how long you will continue treatment with Zepzelca.
- Your healthcare provider may do certain tests during your treatment with Zepzelca to check you for side effects, and to see how well you respond to the treatment.
What should I avoid while using Zepzelca?
- Avoid eating or drinking grapefruit, or products that contain grapefruit juice during treatment with Zepzelca.
What are the possible side effects of Zepzelca?
Zepzelca can cause serious side effects, including:
- Low blood cell counts. Low blood counts including low neutrophil counts (neutropenia) and low platelet counts (thrombocytopenia) are common with Zepzelca, and can also be severe. Some people with low white blood cell counts may get fever, or an infection throughout the body (sepsis), that can cause death. Your healthcare provider should do blood tests before you receive each treatment with Zepzelca to check your blood cell counts.Tell your healthcare provider right away if you develop:
- fever or any other signs of infection
- unusual bruising or bleeding
- tiredness
- pale colored skin
- Liver problems. Increased liver function tests are common with Zepzelca, and can also be severe. Your healthcare provider should do blood tests to check your liver function before you start and during treatment with Zepzelca.Tell your healthcare provider right away if you develop symptoms of liver problems including:
- loss of appetite
- nausea or vomiting
- pain on the right side of your stomach-area (abdomen)Your healthcare provider may temporarily stop treatment, lower your dose, or permanently stop Zepzelca if you develop low blood cell counts or liver problems during treatment with Zepzelca.
The most common side effects of Zepzelca include:
- tiredness
- low white and red blood cell counts
- increased kidney function blood test (creatinine)
- increased liver function blood tests
- increased blood sugar (glucose)
- nausea
- decreased appetite
- muscle and joint (musculoskeletal) pain
- low level of albumin in the blood
- constipation
- trouble breathing
- low levels of sodium and magnesium in the blood
- vomiting
- cough
- diarrhea
These are not all of the possible side effects of Zepzelca. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1‑800‑FDA‑1088.
General information about the safe and effective use of Zepzelca
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information guide. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about Zepzelca that was written for health professionals.
What are the ingredients in Zepzelca?
Active ingredient: lurbinectedin
Inactive ingredients: sucrose, lactic acid and sodium hydroxide.
Label
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
- NDC 68727-712-01
ZEPZELCA
(lurbinectedin)
for injection
4 mg per vial
FOR INTRAVENOUS INFUSION ONLY
Reconstitute before further dilution.
Each single-dose vial contains 4 mg of lurbinectedin
as a sterile lyophilized powder
Rx Only
Single-dose vial
Discard unused portion.
Caution: Cytotoxic agent
