Zokinvy
Generic name: lonafarnib
Drug class: Miscellaneous metabolic agents
Medically reviewed by A Ras MD.
What is Zokinvy?
Zokinvy is a prescription medicine used to lower the risk of death in adults and children 12 months of age or older with Hutchinson-Gilford Progeria Syndrome (HGPS), who have a certain body surface area.
- It is also used to treat adults and children 12 months of age or older with certain types of Progeroid Laminopathies, who have a certain body surface area.
Zokinvy is not for use in people with non-HGPS Progeroid Syndromes or with Progeroid Laminopathies that are processing-proficient.
It is not known if Zokinvy is safe and effective in children under 12 months of age.
Description
ZOKINVY (lonafarnib) is a farnesyltransferase inhibitor. The chemical name for lonafarnib is 4-[2-[4-[(11R)-3,10-dibromo-8-chloro-6,11-dihydro-5H- benzo[1,2] cyclohepta [2,4-b]pyridin-11-yl]piperidin-1-yl]-2- oxoethyl]piperidine-1-carboxamide. Its molecular formula is C27H31Br2ClN4O2, molecular mass is 638.8 g/mol, and its chemical structure is depicted below.
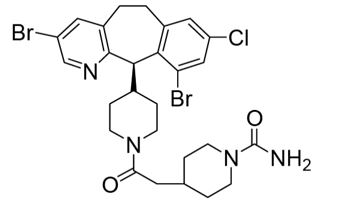
ZOKINVY (lonafarnib) capsules for oral administration contain 50 mg or 75 mg of lonafarnib as the active ingredient and the following inactive ingredients: croscarmellose sodium, magnesium stearate, poloxamer 188, povidone, and silicon dioxide. The capsule shells of both strengths contain gelatin, titanium dioxide, and yellow iron oxide; the 75 mg capsule also contains red iron oxide. The imprinting ink contains ammonia solution, black iron oxide, butyl alcohol, dehydrated alcohol, isopropyl alcohol, potassium hydroxide, propylene glycol, purified water, and shellac.
Mechanism of Action
Lonafarnib inhibits farnesyltransferase to prevent farnesylation and subsequent accumulation of progerin and progerin-like proteins in the inner nuclear membrane.
Who should not take Zokinvy?
Do not take Zokinvy if you are taking:
- a strong or moderate CYP3A inhibitor or inducer
- midazolam
- lovastatin or simvastatin or atorvastatin
Ask your healthcare provider if you are not sure if your medicine may be one that is listed above.
What should I tell my healthcare provider before taking Zokinvy?
Before taking Zokinvy, tell your healthcare provider about all of your medical conditions, including if you:
- have kidney problems.
- have eye problems.
- are pregnant or plan to become pregnant. Zokinvy can harm your unborn baby. Females who are able to become pregnant should use effective birth control (contraception) during treatment with Zokinvy.
- are breastfeeding or plan to breastfeed. It is not known if Zokinvy can pass into your breast milk. Talk to your healthcare provider about the best way to feed your baby during treatment with Zokinvy.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Taking Zokinvy with certain other medicines may affect how the other medicines work and other medicines may affect how Zokinvy works, and may increase your risk of side effects.
Know the medicines you take. Keep a list of them with you to show your healthcare provider and pharmacist when starting a new medicine.
How should I take Zokinvy?
- Take Zokinvy exactly as your healthcare provider tells you to.
- Do not stop taking Zokinvy without talking to your healthcare provider. Your healthcare provider my change your dose of Zokinvy if needed.
- Your healthcare provider will provide you with the dose you should be taking. The dose is based on body size (height and weight).
- Take Zokinvy 2 times a day with morning and evening meals.
- Swallow Zokinvy capsules whole with an adequate amount of water. Do not chew Zokinvy capsules.
- If you or your child cannot swallow the Zokinvy capsule whole, see the detailed Instructions for Use that comes with Zokinvy for information about how to prepare and give or take a dose of Zokinvy by mixing the contents of a Zokinvy capsule with either Ora Blend SF, Ora-Plus, orange juice, or applesauce.
- Each dose of Zokinvy mixture must be prepared fresh and taken within about 10 minutes of mixing.
- Do not take Zokinvy with any juice that contains grapefruit or Seville oranges. Seville oranges may also be called bitter or sour oranges.
- If you miss a dose of Zokinvy, take it as soon as possible up to 8 hours before your next scheduled dose with food. If it is less than 8 hours before your next dose of Zokinvy, skip the missed dose and take Zokinvy at your next regularly scheduled dose.
What are the possible side effects of Zokinvy?
Zokinvy may cause serious side effects, including:
Severe kidney problems, Zokinvy may cause severe kidney problems. Your healthcare provider will monitor your kidney function regularly during treatment with Zokinvy.
Eye problems. Zokinvy may cause problems with night vision. Call your healthcare provider right away if you develop any new changes in your vision.
The most common side effects of Zokinvy include:
- vomiting
- diarrhea
- infection
- nausea
- decreased appetite
- tiredness
- upper respiratory tract infection
- stomach-area (abdominal) pain
- muscle and joint (musculoskeletal) pain
- electrolyte abnormalities
- decreased weight
- headache
- myelosuppression
- increased liver enzyme blood test results
- decreased blood bicarbonate
- cough
- hypertension
Call your healthcare provider if you continue to have nausea, vomiting or diarrhea that leads to loss of appetite and weight loss during your treatment with Zokinvy.
Zokinvy may cause an increase in your blood pressure (hypertension). Your healthcare provider will check your blood pressure during treatment with Zokinvy. Call your healthcare provider right away if you develop any symptoms of high blood pressure including: headaches, shortness of breath, nosebleeds, flushing, tiredness, dizziness, vision problems, irregular heartbeat, or chest pain.
Zokinvy may cause fertility problems in females and males, which may affect your ability to have children. Talk to your healthcare provider if you have concerns about fertility.
These are not all of the possible side effects of Zokinvy.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. You may also report side effects to Eiger BioPharmaceuticals at 1-833-267-0545.
General information about the safe and effective use of Zokinvy
Medicines are sometimes prescribed for purposes other than those listed in the Patient Information. Do not use Zokinvy for a condition for which it was not prescribed. Do not give Zokinvy to other people, even if they have the same symptoms that you have. It may harm them. You can ask your healthcare provider or pharmacist for information about Zokinvy that is written for health professionals.
How should I store Zokinvy?
- Store at room temperature between 68°F to 77°F (20°C to 25°C)
Keep Zokinvy and all medicines out of reach of children.
What are the ingredients in Zokinvy?
Active ingredient: lonafarnib
Inactive ingredients: croscarmellose sodium, magnesium stearate, poloxamer 188, povidone, and silicon dioxide.
The capsule shell (Zokinvy 50 mg hard capsule) contains: gelatin, titanium dioxide and yellow iron oxide
The capsule shell (Zokinvy 75 mg hard capsule) contains: gelatin, titanium dioxide, yellow iron oxide and red iron oxide.
The printing ink contains: ammonia solution, black iron oxide, butyl alcohol, dehydrated alcohol, isopropyl alcohol, potassium hydroxide, propylene glycol, purified water, and shellac.
For more information call Eiger BioPharmaceuticals at 650-282-6138 or visit www.zokinvy.com or www.eigerbio.com.
Instructions for use for Zokinvy
Zokinvy (ZO-kinvy)
(lonafarnib)
capsules, for oral use
This Instructions for Use contains information on how to mix and give or take a dose of Zokinvy if the capsules cannot be swallowed whole.
Read this Instructions for Use before you start giving or taking Zokinvy and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about you or your child’s medical condition or treatment
Supplies needed to prepare and give or take a dose of Zokinvy
Before mixing a dose of Zokinvy, gather the following supplies:
- the prescribed number of Zokinvy capsules for you or your child’s dose. Place the capsule or capsules on a clean flat surface.
- either Ora-Blend SF, Ora-Plus, orange juice or applesauce for mixing. Do not mix with juice that contains grapefruit or Seville oranges. Seville oranges may also be called bitter or sour oranges.
- if mixing with Ora-Blend SF, Ora-Plus or orange juice: a clean medicine cup with 5 milliliter (mL) and 10 mL measurement levels,
OR
if mixing with applesauce: a clean teaspoon.
- if mixing with Ora-Blend SF, Ora-Plus or orange juice: a clean medicine cup with 5 milliliter (mL) and 10 mL measurement levels,
- clean cup(s) for each Zokinvy capsule to be mixed.
- a clean spoon for stirring the mixture.

How to open Zokinvy capsules and mix the capsule contents
Step 1: If mixing with Ora-Blend SF, Ora-Plus or orange juice: Use a clean medicine cup to measure either 5 mL or 10 mL of Ora-Blend SF, Ora-Plus or orange juice.
If mixing with applesauce: measure either 1 or 2 teaspoonfuls of applesauce.
You can choose to use 5 mL or 10 mL of liquid or 1 or 2 teaspoonfuls of applesauce (See Figure A).

Step 2: Place the Ora-Blend SF, Ora-Plus, orange juice or applesauce measured in Step 1 into a clean cup

Step 3: Hold a Zokinvy capsule above the clean cup containing the liquid or applesauce. Hold the Zokinvy capsule on both sides between your thumb and forefinger. Gently twist and pull apart the capsule (See Figure C). Empty the contents of the capsule directly into the clean cup (See Figure D).

Step 4: Using a clean spoon, mix the Zokinvy capsule contents well (See Figure E). If only 1 capsule is to be taken, skip to Step 6. If 2 capsules are to be taken, go to Step 5.

Step 5: If 2 capsules will be taken, repeat Steps 1 through 4 for the second capsule. After completing the mixing process for the second capsule, the 2 servings can either be placed together in a single cup or remain in 2 serving cups for you or your child to take the full dose of Zokinvy. After you finish, go to Step 6.
Step 6: Give or take all of the Zokinvy mixture with morning and evening meals within about 10 minutes of preparing.

How should I store Zokinvy?
- Store at room temperature between 68°F to 77°F (20°C to 25°C)
Keep Zokinvy and all medicines out of reach of children.
Label
PRINCIPAL DISPLAY PANEL – NDC: 73079-050-30 – 50 MG 30-COUNT BOTTLE LABEL

PRINCIPAL DISPLAY PANEL – NDC: 73079-050-30 – 50 MG 30-COUNT BOTTLE CARTON LABEL
PRINCIPAL DISPLAY PANEL – NDC: 73079-075-30 – 75 MG 30-COUNT BOTTLE LABEL
